PSM Releases Overview of Unapproved Cancer Medication Cases
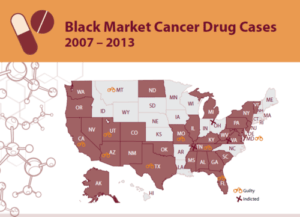 PSM’s Black Market Cancer Drug Cases 2007-2013 looks back through the past 6 years at the numerous cases of fake or misbranded cancer drugs that were sold by shady black market drug sellers and purchased by doctors and clinics throughout the United States.
PSM’s Black Market Cancer Drug Cases 2007-2013 looks back through the past 6 years at the numerous cases of fake or misbranded cancer drugs that were sold by shady black market drug sellers and purchased by doctors and clinics throughout the United States.
Since 2007, 16 physicians and drug distributors have been prosecuted for their roles in exposing U.S. cancer patients to non-FDA approved medication. In many cases clinics purchased the misbranded drugs from questionable suppliers that advertised via “blast faxes” that offered to-good-to-be-true prices on variety of injectable cancer medications.
Since 2011 alone, the FDA has warned approximately 145 medical practices in 29 states that they have done business with a suspect black marketer suspected of distributing counterfeit or unapproved cancer medication. When possible, the FDA tested suspected cancer drugs and found that they contained salt water and acetone instead of the life-saving ingredient.
To learn more about the recent history of counterfeit and misbranded cancer medications and their infiltration of the U.S. drug supply, please download Black Market Cancer Drug Cases 2007-2013.