On February 26, 2026, PSM was glad to see the Indiana legislature pass SB 282, a bill that strengthens compounding and medical spa regulation to keep Indiana patients safer.
As Europol seized a huge amounts of counterfeit pharmaceuticals abroad, a Kentucky legislator proposed a bill to regulate med spas after a constituent was seriously injured and adverse events related to weight loss drugs see surges across the US.
Washington State investigators found serious violations at a Mochi Health-affiliated compounding pharmacy producing GLP-1s, and a med spa bill in Indiana hears public testimony about a med spa bill.
Industry experts told us that these two companies are on the FDA’s Green List, despite problematic inspections in late 2024 and early 2025. Learn what the inspectors found.
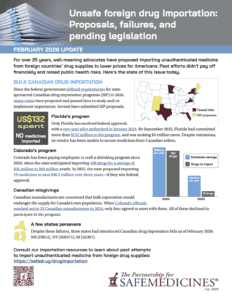
Well-meaning advocates continue to propose importing medicine from foreign countries to lower drug prices for Americans. Past efforts didn’t pay off financially and raised public health risks. Here’s the state of this issue today.
A Pennsylvania compounding pharmacy received another FDA warning letter following a series of enforcement actions, including a $1 million state fine in October.
The last two months of 2025 saw thousands of shipments of drugs popularized by social media imported into the U.S., as well as Xarelto and antibiotics. The shipments carried a wide range of product codes; some appropriate, and others far less so.
Our podcast covers the latest in pharma crime and medicine safety.
Like your information on video? Subscribe to our YouTube playlist!

A recent FDA warning letter highlights upstream supply chain vulnerabilities.

Regulatory confusion leaves med spa patients at risk. Learn more.

Gaps in FDA oversight of 503B compounding facilities put patients at risk.
Click the images below to see more recent videos.