Neurology Associates of Greenville Sentenced in Misbranded Botox Case
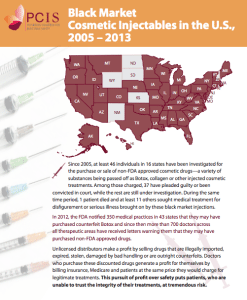 South Carolina medical practice prosecuted for buying non-FDA approved injectable cosmetic treatments.
South Carolina medical practice prosecuted for buying non-FDA approved injectable cosmetic treatments.
A medical practice in Greenville, South Carolina has been sentenced to 3 years of federal probation for treating patients with non-FDA approved Botox, a Department of Justice (DOJ) press release reported on the occasion of the sentencing.
According to the DOJ, “Records obtained during this investigation show that the practice from 2010-2013 purchased non-FDA approved Botox from a wholesaler who purchased the Botox from a factory in Ireland.”
Neurology Associates of Greenville was originally charged “in a 1-count Information with dispensing misbranded drugs, a violation of Title 21, United States Code, Section 331(c),” according to a DOJ press release at the time.
The DOJ also reports that Neurology Associates of Greenville also settled a civil action brought by the Federal Government and paid $300,000 in fines under the civil False Claim Act.
WSPA in Greenville spoke to an area plastic surgeon on the occasion of the sentencing. “Plastic surgeons said they see how something like this could happen because practices are dependent and trustful of drug representatives. However, they said the doctor has an obligation to his patients to ask the right questions.”
“In a world that’s so busy, it would be possible, I think, to hear this and not verify that it’s FDA approved, so we have to be vigilant about it,” Dr. Barry Bishop of Plastic Surgery Associates in Greenville told WSPA.
Agents of the Federal Bureau of Investigations and the Food and Drug Administration of the Health and Human Services investigated the case against Neurology Associates of Greenville. Assistant United States Attorney William J. Watkins, Jr. of the Greenville office prosecuted the case.