National Boards of Pharmacy Finds only 4.2% of Online Pharmacies Are Safe
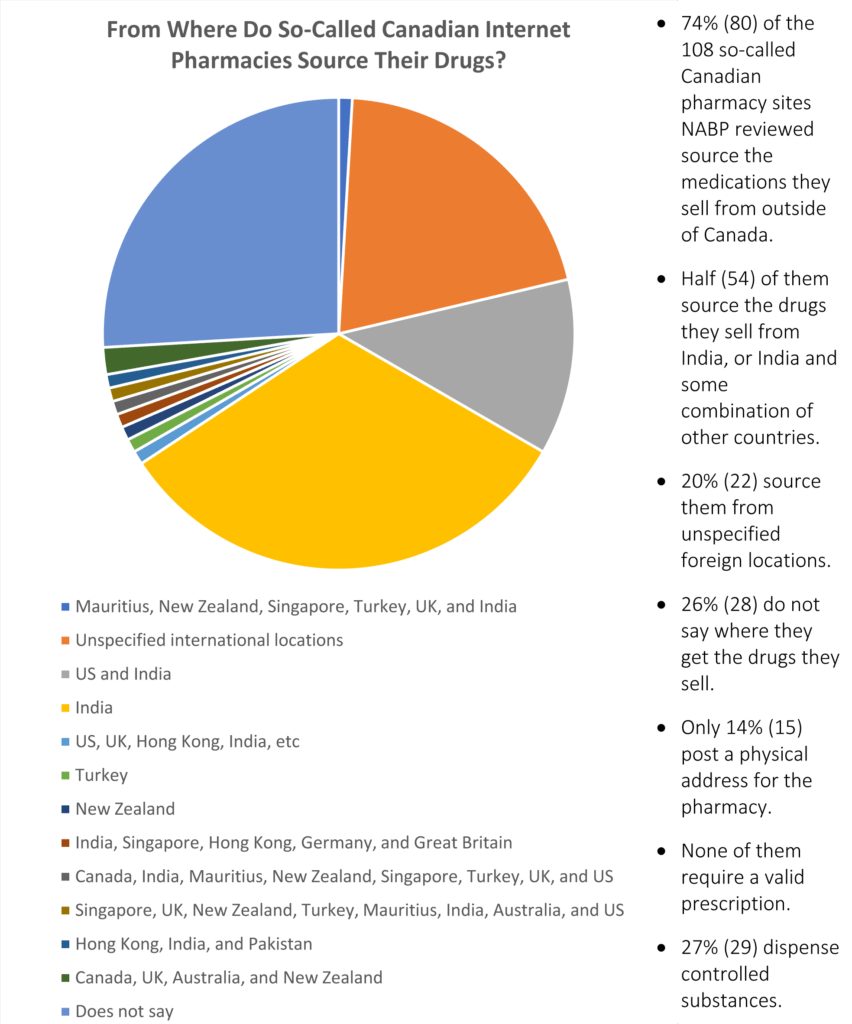
Chart showing the source of medications sold via Canadian online pharmacies, from Internet Drug Outlet Identification Program Progress Report for State and Federal Regulators: August 2017
The National Association of Boards of Pharmacy (NABP) has just released their Internet Drug Outlet Identification Program Progress Report for State and Federal Regulators: August 2017, outlining the current state of fake online pharmacies that sell to U.S. patients.
The NABP states that its mission is to not only assist its member pharmacy boards and jurisdictions in safeguarding public health, but also, they remain “committed to upholding the integrity of the practice of pharmacy – in any practice setting or location – and ensuring that patients worldwide have access to safe and effective prescription medications.”
Out of 11,688 Internet drug outlets reviewed, the NABP found that 11,142 (95.8%) were “operating out of compliance with state and federal laws and/or NABP patient safety and pharmacy standard practices.”
The NABP also examined so-called Canadian online pharmacies. They classified these websites as Canadian online pharmacies if they use “Canada” or “Canadian” in their business title or url, or if they list a Canadian address and offer to sell prescription medication to U.S. customers.
Of the 108 “Canadian” online pharmacies the NABP examined, 74% admitted to sourcing their medication from outside Canada. The remaining 28 sites examined did not offer any information about where their medication came from.
50% of the “Canadian” online pharmacies the NABP looked at shipped medications that they purchase from India, or a mixture of drugs sourced from India and other countries, such as Hong Kong and Singapore.
At it’s opening, the NABP report expresses their concern about what current drug importation proposals could mean for American patients: ‘Many online drug sellers display the Canadian maple leaf as a symbol of the safety and reliability of medications approved for sale in Canada. The drugs they sell to customers outside of Canada, however, are often something altogether different. Since the subject of importing prescription medicine from Canada has made a reappearance in the halls of Congress in recent months, many health care regulators and patient safety advocates have voiced their opposition to importation, stating that such policy would open the floodgates for unapproved and counterfeit medications of unknown origins to enter the United States medication supply chain.’
The NABP report also notes that their concern about pending drug importation legislation in Congress is so profound, that the NABP wrote to Congress in February of this year to extol the legislators resist the temptation of opening up the secure drug supply chain to imported medication.
“In NABP’s nearly 20 years of experience in verifying internet pharmacies, US consumers buying medications from Canadian online pharmacies rarely, if ever, receive the Health Canada-approved products afforded to Canadian customers. Instead, these Canadian pharmacy websites sell US patients medicines manufactured in places where buyers would not even drink the water, eg, India, Turkey, or Southeast Asia.”