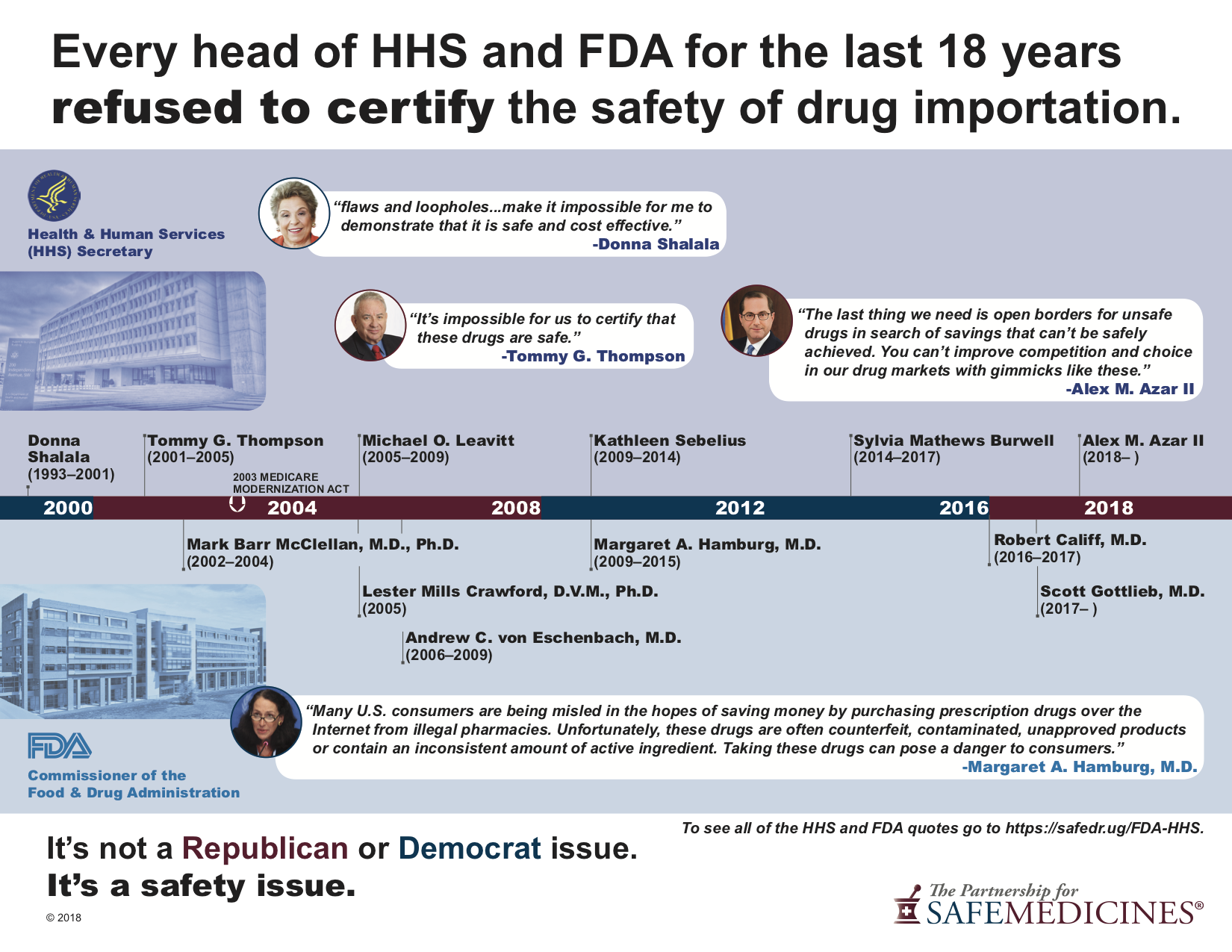
Ordering prescription drugs from non-FDA approved foreign sources is a dangerous path, opposed by all of the previous heads of the United States Food and Drug Administration (FDA), United States Department of Health and Human Services (HHS) since 2000.
Alex M. Azar, II, current Secretary of Health and Human Services, May 2018
"...the last four FDA commissioners have said there is no effective way to ensure drugs coming from Canada really are coming from Canada, rather than being routed from, say, a counterfeit factory in China. The United States has the safest regulatory system in the world. The last thing we need is open borders for unsafe drugs in search of savings that cannot be safely achieved."
Scott Gottlieb, current FDA Commissioner, March 2016
"Providing a reasonable measure of oversight to reduce the number of counterfeits coming through an importation scheme is complex and costly. It’s very hard to “inspect in” safety after a drug is manufactured. There’s no question that a drug importation scheme will increase the flow of counterfeits in the U.S. supply chain."
Dr. Robert Califf, former FDA Commissioner, in November 2015
“FDA would not be able to make safety and quality determinations for prescription drugs offered for import into the United States that have not gone through the U.S. regulatory process. … FDA evaluation of non-FDA-approved imported drugs revealed that while nearly half of imported drugs claimed to be Canadian or from Canadian pharmacies, 85 percent of such drugs were actually from different countries. … Authorizing importation would compromise the closed drug distribution system in the United States and undermine these laws, thus making it easier for unapproved drugs, which may include counterfeit or other substandard drugs, to reach American patients putting their treatment at risk. … FDA is concerned that the risks of unapproved products from foreign sources outweigh any potential cost savings. We are also concerned that adverse events flowing from importation of such unapproved products could lead to diminished confidence in FDA- approved products.”
Dr. Margaret Hamburg, former FDA Commissioner, in November 2009
“Many U.S. consumers are being misled in the hopes of saving money by purchasing prescription drugs over the Internet from illegal pharmacies. Unfortunately, these drugs are often counterfeit, contaminated, unapproved products or contain an inconsistent amount of active ingredient. Taking these drugs can pose a danger to consumers.”
Kathleen Sebelius, former Secretary of Health and Human Services, in April 2009
“I think it [importation] can only be done if the inspections are accomplished and that we are satisfied that the standards are equivalent or above American standards. And that is going to take some time and some work. The secretary has had the authority from Congress, if deemed safe, to engage in re-importation. As you know, in the past, that has not occurred. And it would be my commitment that that would not occur until I was satisfied, if I am confirmed as secretary, that that safety issue is absolutely met.”
Dr. Andrew Von Eschenbach, former FDA Commissioner, in January 2008
[W]e have been unable to be certain we could assure that, even when they are labeled as having come from, what we would consider a reputable source like Canada, the product itself often is not, and is often coming from places other than Canada that we have absolutely no control or confidence in, or when analyzed are found to either not contain the active ingredient or to contain ingredients that are in effect harmful. We have no way of being able to assure the safety of reimports.”
Michael Leavitt, former Secretary of Health and Human Services, in May 2007
“Allowing the importation of drugs outside the current safety system would pose an immediate and significant risk to the public health in the United States.”
Dr. Lester Mills Crawford, former FDA Commissioner, in March 2005
"Reimported and imported foreign medications are currently outside the regulatory system overseen by FDA and State Pharmacy Boards. Therefore, it is very difficult for a consumer to tell if they are getting a medication that meets FDA's strength, quality and purity standards. We have confiscated a significant amount of suspicious packages from foreign sources, many which have contained drugs that don't meet FDA standards. As the government agency responsible for the safety and efficacy of medications, we are greatly alarmed that these drugs are bypassing established safeguards and finding their way into the system."
Dr. Mark McClellan, former FDA Commissioner, in June 2003
"In FDA's experience, many drugs obtained from foreign sources that either purport to be or appear to be the same as U.S.--approved prescription drugs are, in fact, of unknown quality. These outlets may dispense expired, subpotent, contaminated or counterfeit product, the wrong or a contraindicated product, an incorrect dose, or medication unaccompanied by adequate directions for use. The labeling of the drug may not be in English and important information regarding dosage and side effects may not be available. In addition, the drugs may not have been packaged and stored under proper conditions to avoid degradation."
Tommy Thompson, former Secretary of Human Health and Services, in June 2001
“Opening our borders as required under this program would increase the likelihood that the shelves of our pharmacies in towns and communities across the nation would include counterfeit drugs, cheap foreign copies of FDA-approved drugs, expired drugs, contaminated drugs, and drugs stored under inappropriate and unsafe conditions. … I can only conclude that the provisions in the MEDS Act will pose a greater public health risk than we face today and a loss of confidence by Americans in the safety of our drug supply.”
Donna Shalala, former HHS Secretary, in December 2000
“I feel compelled to inform you that the flaws and loopholes contained in the reimportation provision make it impossible for me to demonstrate that it is safe and cost effective.”