New York Ophthalmologist Settles Civil Case Involving Use Of Non FDA-Approved Drugs
As reported by Garden City Patch, an ophthalmologist who lives and works in the city settled civil fraud claims with the federal government for importing non Food and Drug Administration (FDA) -approved medications and using them on his patients. Dr. Mark Fleckner used aflibercept (“Eylea”) and ranibizumab (“Lucentis”) on patients experiencing age-related macular degeneration, as well as other diseases and conditions of the eyes. However, Fleckner submitted claims for reimbursement to Medicare as if he had been using FDA-approved drugs. As part of the settlement, Fleckner must pay $6,955,240.80 in restitution. Non FDA-approved drugs have been found to contain a variety of substances one would not want in their medicine including heavy metals, actual poisons, and substances such as road paint, brick dust, and floor wax.
An investigation by the government revealed that from at least July 1, 2014 to June 27, 2017, Fleckner purchased unapproved medicines because they were less expensive. Medicare reimburses physician-administered drugs at a set rate and because Dr. Fleckner was administering cheaper, foreign-sourced drugs, he was able to pocket the difference between how little he had paid for the drugs and how much more Medicare reimbursed him. This settlement is not an admission of wrongdoing by Dr. Fleckner.
In a press release from the U.S. Department of Justice, U.S. Attorney Richard P. Donoghue made this statement when the settlement was announced: “Dr. Fleckner bypassed the FDA’s regulatory authority by purchasing and administering unapproved pharmaceutical products in violation of Medicare regulations. The settlement holds Dr. Fleckner accountable for his actions and ensures that Medicare funds will only be used for FDA-approved pharmaceuticals.” Special Agent in Charge Jeffrey J. Ebersole of the FDA’s Office of Criminal Investigations added: “FDA’s oversight on prescription drugs protects consumers from illicit medicines obtained from unauthorized foreign sources. We will continue to pursue and bring to justice those who place profits over their patients’ safety.” Former Assistant U.S. Attorney Kenneth M. Abell of the Department of Justice’s Civil Division handed this investigation.
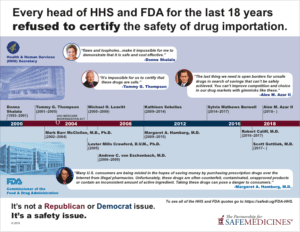
It is not legal for doctors to use non FDA-approved products on their patients, and the debate about allowing drug importation is nothing new. Every single commissioner of the FDA or secretary of Health & Human Services for the past 18 years has said that if we allow drug importation, safety cannot be guaranteed. You can read their statements here.