Oregon Woman Sentenced For Injecting Patients With Foreign-Sourced Botox and Juvéderm
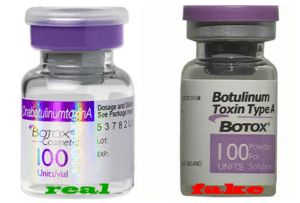
Source: The Counterfeit Report
The Oregonian ran an article that announced the sentencing of a former doctor who injected patients in her home with wrinkle-reducing beauty treatments illegally purchased from foreign sources. PSM previously discussed this case when Brenda Roberts pleaded guilty in June 2018. A judge sentenced Brenda Roberts to six months probation and 40 hours of community service.
In a press release announcing the sentencing from the U.S. Department of Justice, the names of three additional online sources used by Roberts were made public. One has already ceased to exist, the second one lacks a secure connection making it easier for bad actors to steal your information, and the third one, AllDayChemist.com, is a known rogue internet pharmacy according to LegitScript. U.S. Attorney Billy J. Williams said, “Purchasing foreign-sourced and unapproved drugs and devices on the internet poses a grave danger to the health and safety patients. Dr. Roberts violated her professional duty to ‘do no harm’ and instead subjected her patients to an incredible risk of injury.”
The U.S. Drug Enforcement Administration (DEA) initially began investigating Roberts for dispensing controlled substances over the Internet. Once agents with the DEA learned that the doctor was administering Botox to patients in her home, they referred the matter over to the U.S. Food and Drug Administration’s Office of Criminal Investigations (FDA-OCI). Special Agent in Charge of the FDA-OCI’s Los Angeles Field office Lisa L. Malinowski stated: “U.S. consumers reply on FDA oversight to ensure that the drugs and medical devices they use are safe and effective. Rogue health care professionals who obtain foreign unapproved medical products, and dispense and administer those products to their patients, put the health of those patients at significant risk. We will continue to pursue and bring to justice those who choose to put the public’s health at such risk.”
Roberts voluntarily surrendered her medical license when she first came under investigation. In addition to pleading guilty in June to the criminal case, Roberts resolved claims via a monetary civil settlement with the U.S. Attorney’s Office of Civil Division in May 2018. FDA-OCI and DEA investigated this case, and Assistant U.S. Attorney Donna B. Maddux prosecuted it.