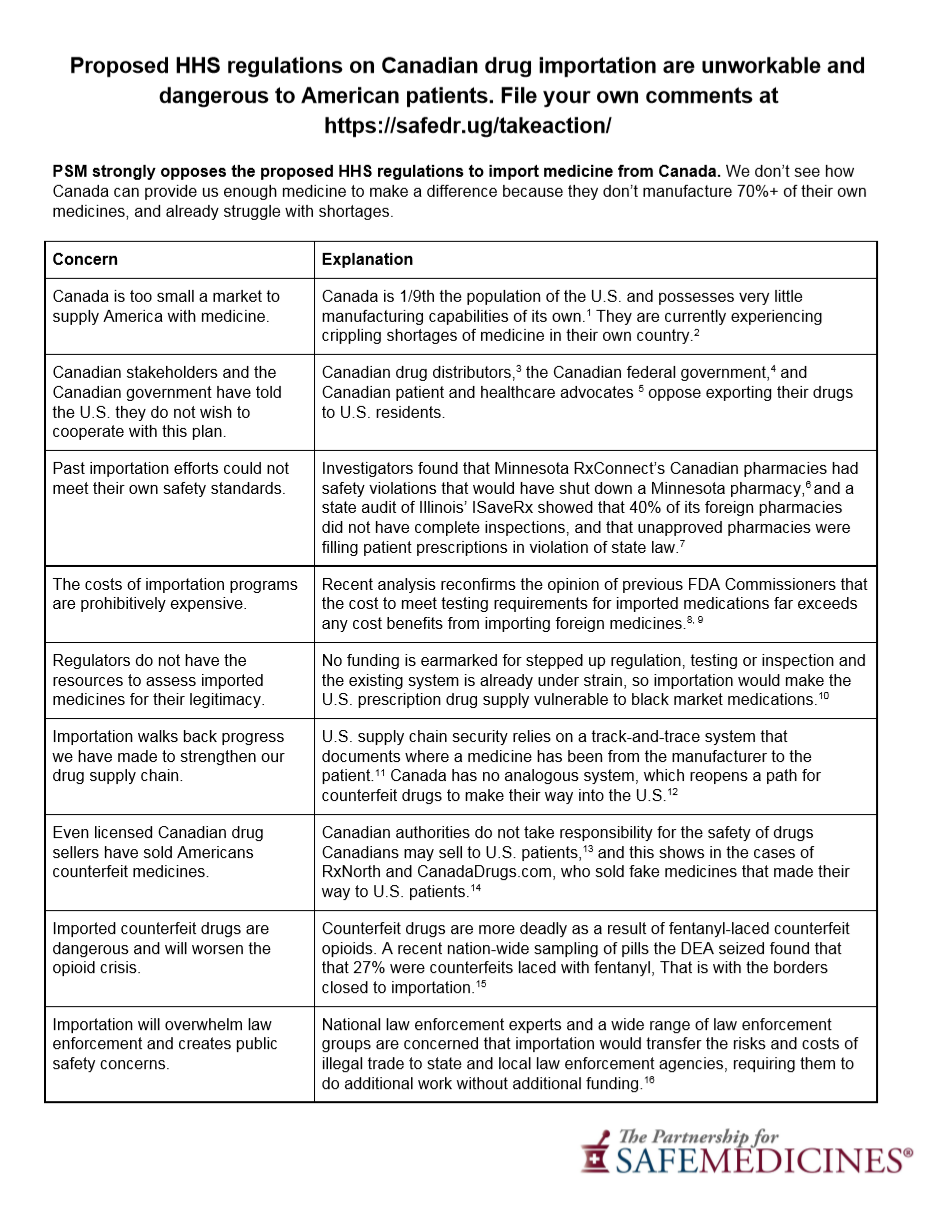
HHS comments come in overwhelmingly against Canadian drug importation proposal
Yesterday ended a 78-day comment period for the White House's proposal to import medicine from Canada. In all, over 1,000 comments were filed. Overwhelmingly, these comments opposed the proposed rule or expressed skepticism that the rule could meet the two requirements listed in the Medicare Modernization Act of 2003: be safe and save consumers money. In fact, when you read the comments, it is clear that this policy is overwhelmingly opposed by experts on the issues of economics and medicine safety.
From a raw count, comments ran 20-to-1 against the proposal. In an amazing development, over 25 different Canadian organizations filed comments against the proposal. Canada has an enormous drug shortage problem and very little manufacturing capacity. These organizations expressed their concern that our country of 350 million people would drain away the medication of their 37 million population very quickly.
Dozens of Canadian patient groups, including Diabetes Canada, Canadian pharmacists, medicine wholesalers, physicians, nurses, and even the Canadian government itself, filed comments against the regulation. Organizations representing healthcare professionals in all ten Canadian provinces filed comments against this rule change. As far as we can tell, not a single comment in favor of the proposal came from anyone in Canada. Can any policy that has universal opposition in Canada ever be implemented?
On the U.S. side, the picture showed staunch opposition as well. Again, comments against this rule change ran more than 20-to-1 against the proposal, and patients, pharmacists, and healthcare experts from 49 out of 50 U.S. states filed comments against the proposal. (Where's the love Wyoming?)
The opposition was abundantly clear from experts who are charged with keeping the drug supply chain safe and actually handling the medications that our families consume daily. Manufacturers and the distributors of medication in both the U.S. and Canada filed comments against this rule change. Many comments cited concerns about safety being undermined and cost savings being impossible to achieve. For historical reference, importation programs promising and failing to deliver safe cost savings have been documented repeatedly in Illinois, Minnesota, and Maine. But we all know what they say about those who cannot remember the past repeating it.
What's more, pharmacists came out in force with their concerns. Pharmacists are on the front lines of delivering healthcare in America, and they also understand both the structural issues with pricing with regards to Pharmacy Benefit Managers, and the gag clauses that until recently, prevented pharmacists from telling their patients when the generic options were cheaper than brand name medicines.
Pharmacists from every walk of life filed comments against the proposal, including pharmacists in community pharmacies, hospitals, nursing homes and other institutions, and even pharmacy professors. In fact, of the four states that started last year to implement importation (Florida, Colorado, Maine, and Vermont) all four state pharmacy association filed comments against the drug importation proposal. Additionally, the pharmacy associations in 15 other states joined them.
Multiple commenters cited issues related to the fact that this proposal would undermine Track-and-Trace, because there is no Track-and-Trace system in Canada. We cannot emphasize this enough; it is impossible to apply Track-and-Trace to medicine bought from a Canadian vendor. There is no way to track it back to the manufacturing floor, as you can with medicine made for the American market. This giant safety hole of importation programs should scare us all.
We've included an infographic that summarizes the key points of the comments to the White House on this rule. We hope that you have some time to let some of the great organizations that filed comments against the rule know that you appreciate them standing up for safety.