Drug Importation in Maine: An Overview
Synopsis of LD 1272:
In June 2019, the Governor of Maine signed LD 1272, a bill which directs the Maine Department of Health and Human Services to design a program for wholesale importation of prescription drugs into Maine from Canada that complies with federal requirements.
Under the Medicare Modernization Act of 2003, Maine is required to submit a plan to HHS to import medicine from Canada that meets requirements set in that legislation.
Current status:
Maine submitted an application to run an importation program to the federal government in April 2020.
We discussed that plan in our May 20, 2020: Maine Importation Update.
How should we evaluate this program?
The program hasn't started yet, so there's no way to measure whether it saved money or kept patients safe, both promises made at the time of passage. However, the 2003 Medicare Modernization Act contains requirements for safety requirements built into any such program.
Official actions and statements
- April 2020: Maine submits an Application to Operate a Section 804 Prescription Drug Importation Program to the federal government
- June 24, 2019: Maine LD 1272 is signed into law
Planning documents
- LD 1272: Text of the Bill | Fiscal Note | Public Hearing Testimony
- State of Maine Canadian Drug Importation Program Considerations (Horvath Health Policy, February 2020 )
News and Commentary
- Response to PSM's Freedom of Access Act Submission for Maine's Request for Comment Period, April 29, 2020
- Henry Miller and John Cohrssen, "Commentary: A Smarter Way to Curb Drug Prices," Kennebec Journal and Morning Sentinel, August 22, 2019.
- Steven Webb, "Maine's Proposal to Bulk Buy Drugs From Canada Not Feasible, Pharmacists Say," CBC, November 20, 2019.
Background / resources
Learn more about
Testimony Opposing the Bill

Past efforts: LD 171
On June 27, 2013, the Maine Legislature passed LD 171, the "Act to Facilitate Personal Importation of Prescription Medication from International Mail Order Prescription Pharmacies," which permitted Mainers to personally import prescription drugs from licensed retail pharmacies in Australia, Canada, Northern Ireland, New Zealand or the United Kingdom.
The legislation provided no oversight or enforcement provisions; if patients received substandard or counterfeit drugs from foreign pharmacies that had lied about their licensing, there was no legal recourse for the patients.
A federal judge struck LD 171 down in February 2015: U.S. District Court Judge Nancy Torresen ruled that the federal government—not Maine—had jurisdiction over importation, and that LD 171 compromised the Federal Food, Drug & Cosmetic Act.
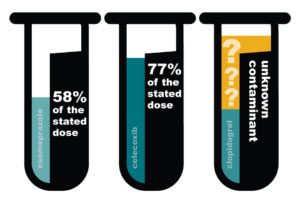
There were also safety concerns: Pharmacy Professor Dr. Kenneth McCall (then president of the Maine Pharmacy Association, and currently a PSM board member) was concerned about the quality of medicines being advertised to Maine residents. In 2014, he ordered three medications from a company called Canada Drug Center. The medicines he received did not come from pharmacies in Australia, Canada, Northern Ireland, New Zealand or the United Kingdom. When he tested them, he found that they were substandard and contaminated.
PSM on LD 171:
By February of 2015 the law had been thrown out, but only after the President of the Maine Pharmacy Association experienced first hand what patients can be exposed to when buying drugs from a Canadian online pharmacy. On June 27, 2013, the Maine Legislature passed LD 171, the Act to Facilitate Personal Importation of Prescription…
Washington, D.C. (February 26, 2015) – Dr. Marv Shepherd, President of the Partnership for Safe Medicines, released the following statement after a federal judge overturned Maine’s dangerous prescription drug importation law: “The easiest way for dangerous and potentially deadly counterfeit medicines to infiltrate our secure supply chain is when they are purchased from illegal and unregulated overseas online…
Board votes unanimously to ask Maine Attorney General to send cease-and-desist letter to a Canadian online pharmacy, and to investigate the charge that they are violating Maine law. CanadaDrugCenter’s activities first came to light when Maine Pharmacy Association President Kenneth McCall filed a complaint against them for selling medication that did not originate in approved…
Kenneth McCall of the Maine Pharmacy Association wanted to find out what he would receive when ordering medication from Canada Drug Center, a company who began advertising in Maine as soon as the new drug importation law was passed. When he saw the drugs were sourced in places not permitted by the new law, he…
Ms. Arnold spoke at Interchange 2013 about the events in her state that resulted in the passage of a controversial law that allows non-FDA approved medication to be imported from unregulated sources into the state of Maine. At the heart of this recent law is a Canadian business called CanaRX that Ms. Arnold stated “came into…