Medication Testing Makes Drug importation Prohibitively Expensive
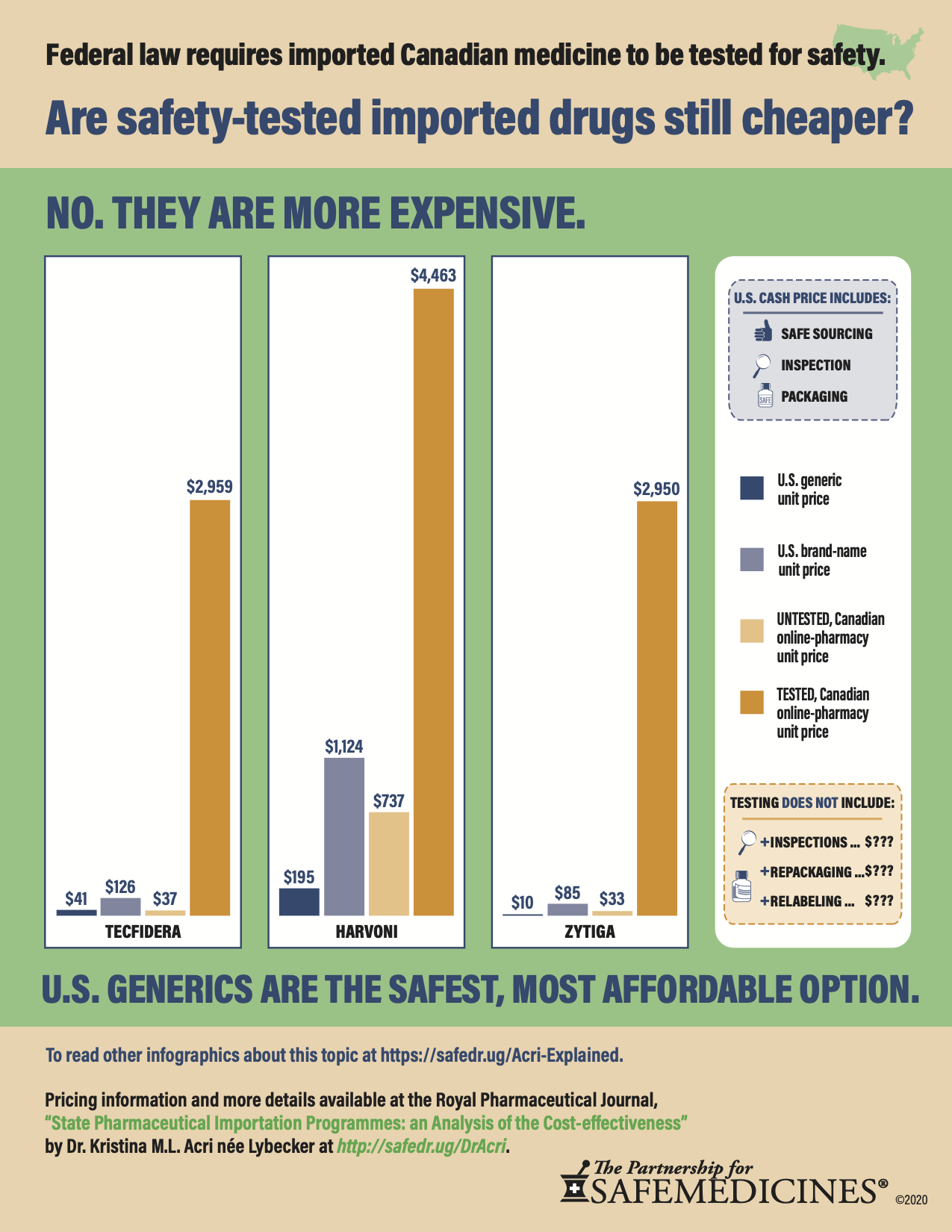
Federal legislation requires that any Canadian drug importation program test a "statistically valid sample" of medicines from each imported batch. This means you have to test more than a single dose or package, but to achieve 99.99% confidence and reliability, that you test thousands or sometimes tens of thousands in a batch.
PSM asked a trained economist with experience in counterfeit medicines, Dr. Kristina Acri, to do a cost analysis of 24 prescription drugs people like to talk about importing from Canada and compute the cost of federally-required safety testing. (See
"State Pharmaceutical Importation Programs: An Analysis of the Cost Effectiveness", JPHSR, May 5, 2020 ) Her research illustrates clearly that a safe drug importation program will be very expensive and not financially feasible. As she said so aptly, "These schemes can be cheap, or they can be safe, but not both."
(Click images to enlarge.)
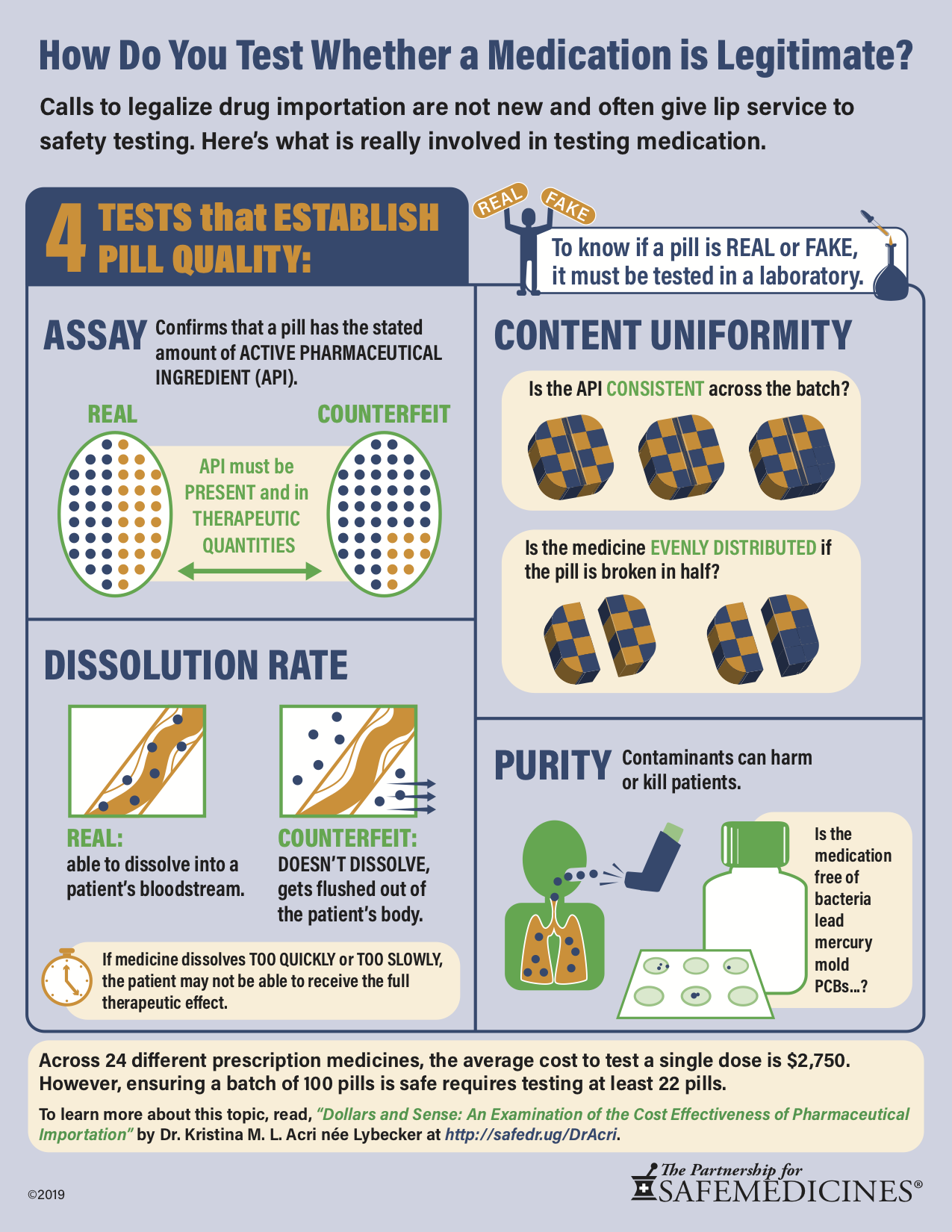
Testing medicine to verify that it is genuine is a complicated process. Across 24 different prescription medicines, the average cost to test a single dose is $2,750.
Ensuring that a batch of 100 pills is safe requires testing at least 22 pills.
Achieving 99.999% certainty requires even more testing, at tremendous expense.
Once you factor in the required testing, U.S. generics are cheaper.
Even testing individual batches of pills to reach 90% certainty will mean one out of every ten pills could be fake. All these tests will eat up any savings that American patients might see from prescription drug importation.
To learn more about this topic, read PSM's summary: safedr.ug/Acri-Explained.