FDA Alert: Dietary Supplement Recalled Due to Presence of Prescription Drug Ingredients
This is a reprint of an FDA Alert.
Gear Isle Issues Voluntary Nationwide Recall of Pro Power Knight Plus, NUX Male Enhancement and DYNAMITE SUPER Capsules Due to the Presence of Sildenafil and Tadalafil
When a company announces a recall, market withdrawal, or safety alert, the FDA posts the company's announcement as a public service. FDA does not endorse either the product or the company.
May 2, 2023
Summary
Company Announcement Date: April 26, 2023
FDA Publish Date: April 26, 2023
Product Type: Drugs, Over-the-Counter Drugs
Reason for Announcement: Product contains undeclared tadalafil and sildenafil
Company Name: Gadget Island, Inc.
Brand Name: Pro Power Knight Plus, NUX, Dynamite Super
Product Description: Male enhancement capsules
Company Announcement
FOR IMMEDIATE RELEASE – April 26, 2023– West Sacramento, CA, Gear Isle is voluntarily recalling the following products listed in the table below to the consumer level. FDA analysis has found the products to be tainted with sildenafil and tadalafil. Sildenafil and tadalafil are ingredients known as Phosphodiesterase Inhibitors (PDE-5) inhibitors found in FDA-approved products for the treatment of male erectile dysfunction. The presence of sildenafil and tadalafil in these products renders them unapproved drugs for which the safety and efficacy have not been established and, therefore, subject to recall.
Risk Statement: Consumption of products with undeclared sildenafil and tadalafil may interact with nitrates found in some prescription drugs (such as nitroglycerin) and may cause a significant drop in blood pressure that may be life threatening. People with diabetes, high blood pressure, high cholesterol, or heart disease often take nitrates. Among the adult male population who are most likely to use these products, adult males who use nitrates for cardiac conditions are most at risk from these products. To date, Gear Isle has not received any reports of illness or adverse events related to this recall.
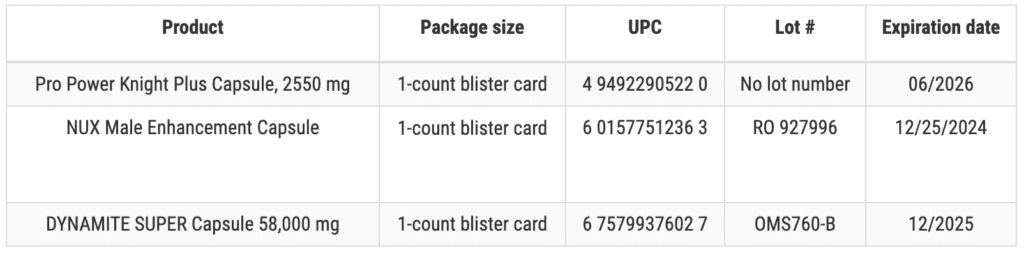
These products are marketed as dietary supplements for male sexual enhancement and are packaged in 1-count blister cards and can also be identified by the images below. The products were distributed Nationwide in the USA via internet sales via the website gearisle.com.
Gear Isle is notifying its customers by recall notification letter via email and press release and is arranging for return and refunds of all recalled products. Consumers who have the products which are being recalled should stop using and return them for a refund.
Consumers with questions regarding this recall can contact Gear Isle customer service by calling 888-387-4753 or emailing infoATgearisleDOTcom on Monday to Friday from 10am to 4pm PST. Consumers should contact their physician or healthcare provider if they have experienced any problems that may be related to taking or using this drug product.
Adverse reactions or quality problems experienced with the use of this product may be reported to the FDA's MedWatch Adverse Event Reporting program either online, by regular mail or by fax.
- Complete and submit the report Online: www.fda.gov/medwatch/report.htm
- Regular Mail or Fax: Download form www.fda.gov/MedWatch/getforms.htm2 or call 1-800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178
This recall is being conducted with the knowledge of the U.S. Food and Drug Administration.
Company Contact Information
Consumers:
Gear Isle Customer Service
888-387-4753
infoATgearisleDOTcom





