Counterfeit and compounded injected diabetes and obesity treatments — twin threats to American patients
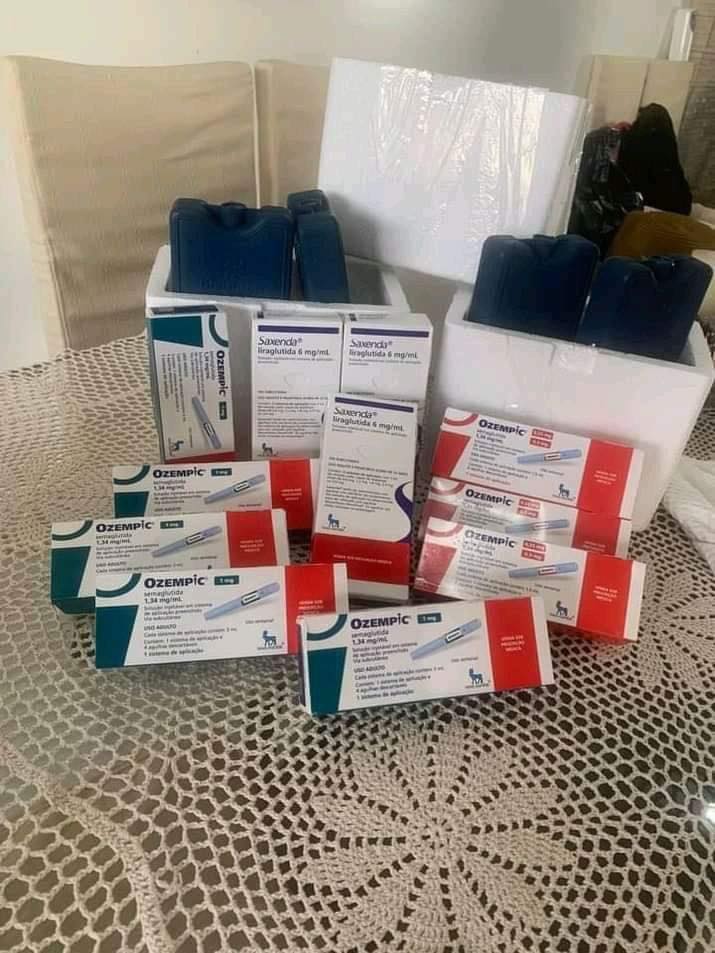
Patients in the United States and around the world who are seeking Ozempic, Mounjaro, Wegovy or Zepbound are encountering a dangerous array of counterfeits as well as compounded semaglutide or tirzepatide falsely marketed as the real thing.
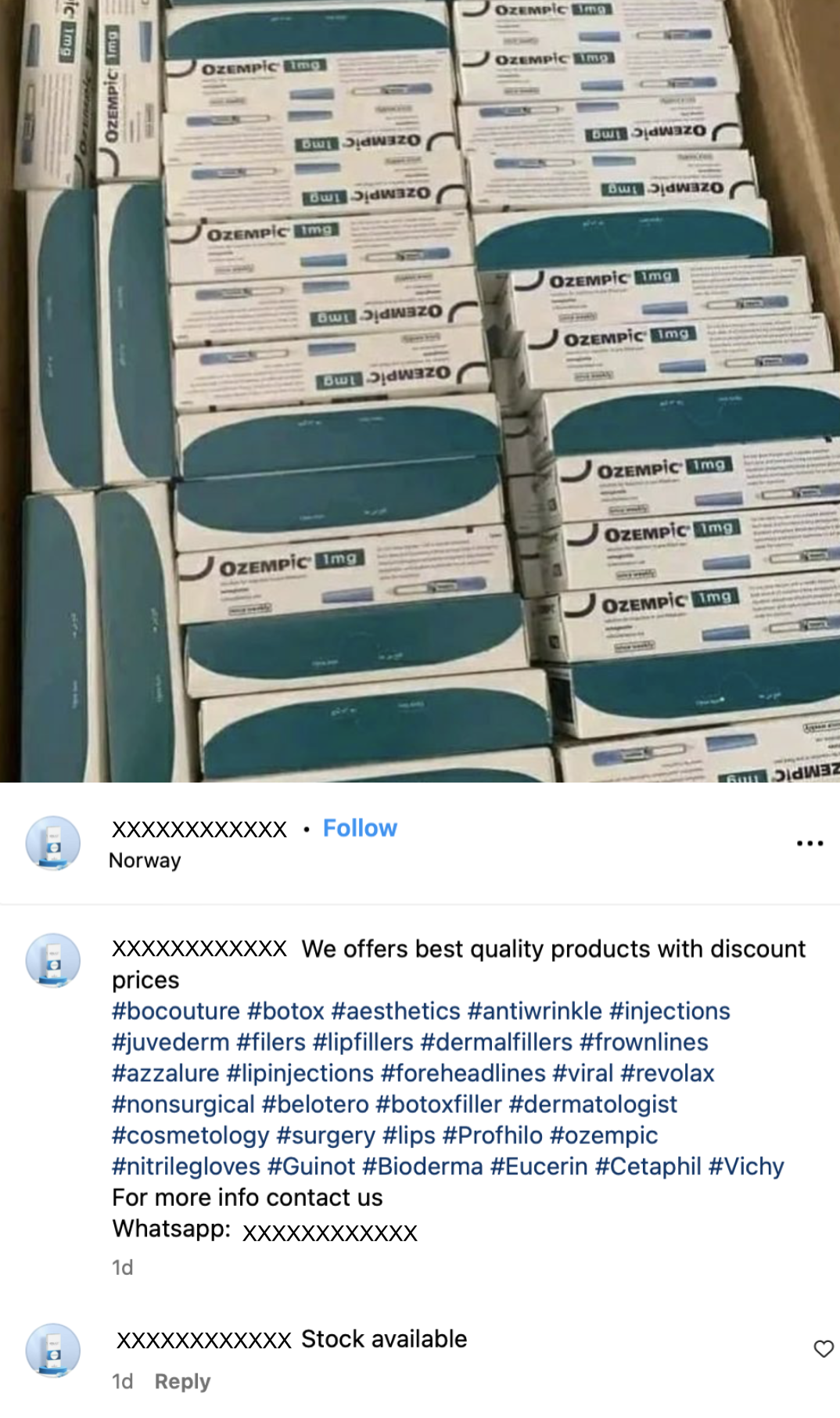
According to a recent report from the National Association of Boards of Pharmacy, these sales are happening alongside an uptick in black market sales of Ozempic and related drugs made with semaglutide, liraglutide, and tirzepatide on social media platforms and online marketplaces.
These unregulated, injected products are a serious threat to patient health.
New from PSM
More on diabetes & weight-loss injectables
- Counterfeits and compounding
- Compounding lawsuits
- What it means that the shortage of weight loss drugs is almost over
More about compounded medicine
Download PSM's guides:
| Compounding harm |
| Weight-loss injectables |
On this page
Fakes in the U.S. and worldwide
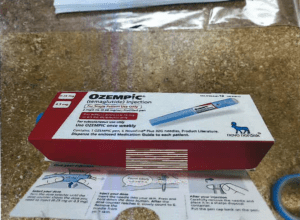
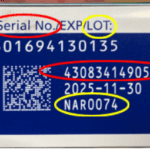
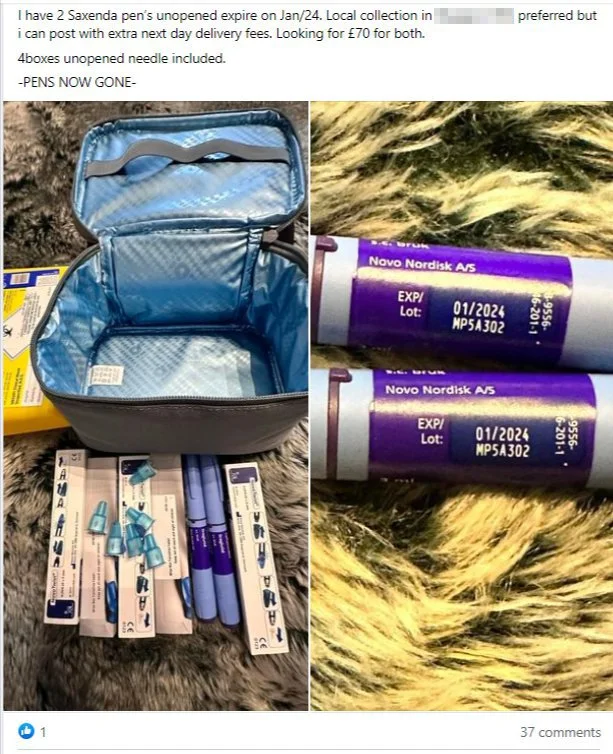
In April 2025 The U.S. Food and Drug Administration announced that it had seized hundreds of units of counterfeit Ozempic in the U.S. drug supply and warned Americans to check lot and serial numbers of any Ozempic in their possession. This followed fake Ozempic found at a pharmacy in Arkansas in late 2024, and the FDA's seizure of "thousands of units" of counterfeit Ozempic pens with counterfeit needles seized in 2023. Because the sterility of the counterfeit needles cannot be confirmed, they present an increased risk of infection for patients using the fake products.

Counterfeit needles seized with fake Ozempic in December 2023 (FDA)
There is good news, though. Because of our federally-mandated track-and-trace system, the Arkansas pharmacy mentioned above and the state's board of pharmacy were able to identify a counterfeit Ozempic pen immediately with the National Association of Boards of Pharmacy's product verification tool, Pulse. The suspect medicine was quarantined and the board revoked the license of the distributor that supplied them.
Images of a fake Ozempic pen quarantined in 2024 (Arkansas State Board of Pharmacy)
Importing Ozempic or other GLP-1s from overseas is also dangerous. Fake versions have been reported in dozens of countries since 2023. Many of these fakes are relabeled insulin pens that have caused hospitalizations for hypoglycemia here and abroad.
More about counterfeits
- FDA warns consumers not to use counterfeit Ozempic (semaglutide) found in U.S. drug supply chain (April 2025 update)
- Falsified OZEMPIC (semaglutide) identified in the WHO Regions of Americas and Europe (WHO Medical Product Alert, June 2024)
- FDA warns consumers not to use counterfeit Ozempic (semaglutide) found in U.S. drug supply chain (December 2023)
- Novo Nordisk's counterfeit Ozempic alert (June 2023)
- How to identify a fake Ozempic pen (from Novo Nordisk's June alert)
- RogueRX activity report: Injectable weight loss drugs: How illegal online drug sellers are taking advantage of patients (NABP, April 2024)
Compounded injectables
Source: Complaint, Novo Nordisk v ProHealth Investments
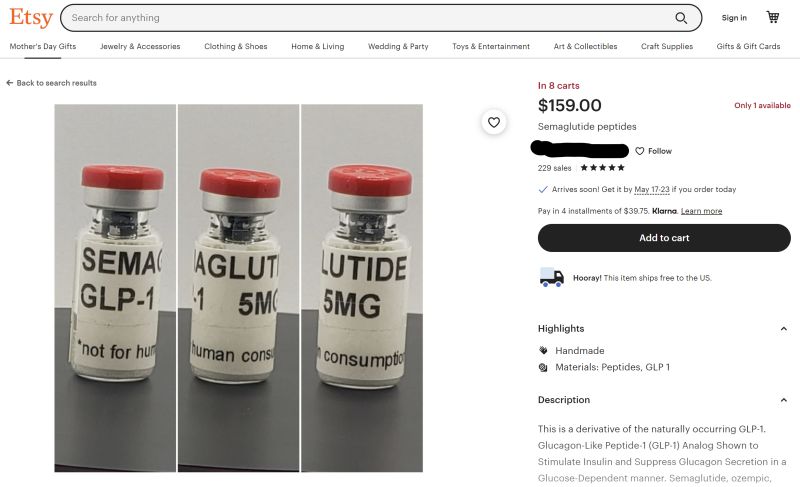
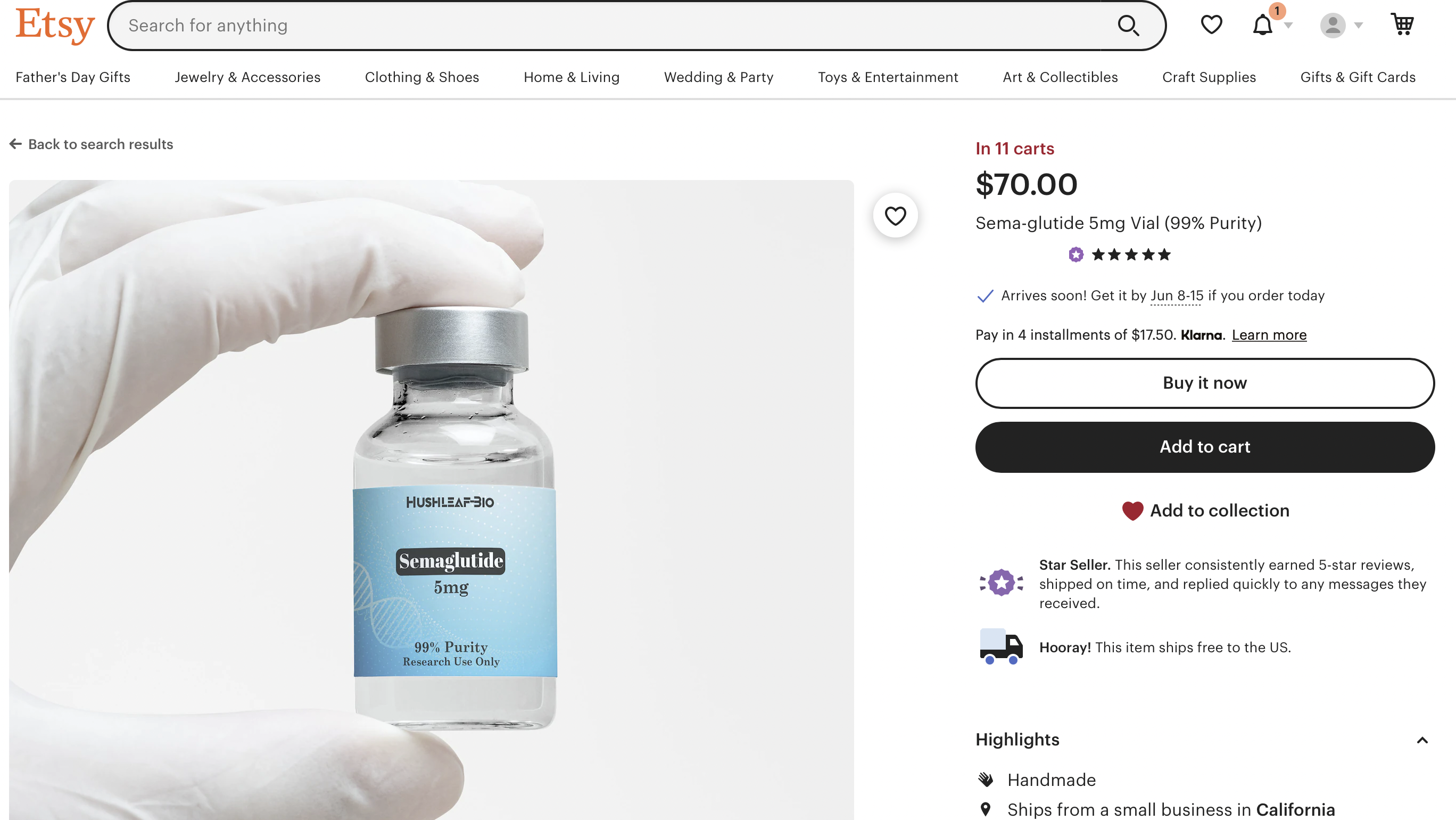
All kinds of clinics, medical spas and wellness-oriented businesses are selling Wegovy, Mounjaro and their sister drugs lately, and most of them are doing it online. Generally, though, these sites aren't selling genuine, FDA-approved Wegovy.
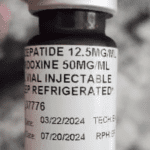
They're selling compounded versions of their active ingredients, semaglutide and tirzepatide.
Compounding pharmacies are permitted to make compounded substitutes for Ozempic, Wegovy, Mounjaro and Zepbound if they are in shortage. However, compounded drugs are not as safe as commercially-marketed ones because the FDA does not review them for effectiveness or quality. It's also more difficult to find the source of problems with compounded medicines, since they aren't tracked as part of the DSCSA.
There are reasons to be wary of these compounded injectables:
- Novo Nordisk and Eli Lilly, the only FDA-approved makers of semaglutide and tirzepatide, have said that they are not supplying compounders. This raises questions about what active ingredients compounded products contain.
- The FDA has warned about reports of adverse events from compounded semaglutide, and has found that some bootleg products contain ingredients such as semaglutide sodium and semaglutide acetate, which haven't been found to be safe or effective.
- In legal filings for trademark violations, Novo and Lilly have reported finding contaminated, counterfeit, and subpotent compounded injections.
- Inspections have found compounding pharmacies operating under poor conditions that have lead to patient illnesses and death.
If you are taking compounded versions of these drugs, please heed the FDA's advice. Only use compounded drugs if FDA-approved medicines are unavailable; buy these medicines from licensed pharmacies or outsourcing facilities with a prescription from a licensed health care provider; and be alert to unexpected side effects that could be the result of unregulated ingredients.
More about compounding concerns
- An open letter from Eli Lilly and Company regarding certain practices related to Mounjaro and Zepbound (June 2024)
- FDA warns about adverse events from compounded semaglutide (May 2023)
- Novo Nordisk escalates legal actions to safeguard patients from potentially harmful compounded “semaglutide” drugs (May 2024)
Novo Nordisk on how to identify a fake Ozempic pen
Genuine:
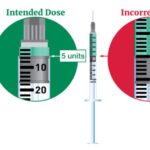
- Genuine Novo Nordisk Ozempic® pens do not extend or increase in length when setting the dose.
- The dose dial window only shows intended doses:
- On the pen intended to deliver 0.25/0.5 mg doses, it only shows -0-, 0.25 and 0.5 once dialed up to the intended doses
- On the pen intended to deliver 1 mg dose, it only shows -0- and 1 mg once dialed up to the intended dose
- On the pen intended to deliver 2 mg dose, it only shows -0- and 2 mg once dialed up to the intended dose
- Authentic Ozempic® pens are currently available in the following configurations:
- 0.25/0.5 mg pen
- 1 mg pen
- 2 mg pen
- The box containing authentic Ozempic® will include 4 needles that attach directly onto the pen, except the Ozempic® 0.25/0.5 mg dose carton, which has 6 needles
Counterfeit:
- A counterfeit pen may be identified based on scale extending out from the pen when setting the dose.
- The label on a counterfeit pen could be of poor quality and may not adhere well to the pen.
- A counterfeit carton may have spelling mistakes on the front of the box (i.e., 1pen and 4 doses without space between ‘1’ and ‘pen’) as seen in photo.
- A counterfeit carton may not include the tamper resistant/perforation.
- The batch number printed on a counterfeit box may not correspond to the product strength stated on the same box and pen.