March 4, 2024: Colorado submits a sharply limited drug importation proposal to the FDA
Major Stories
Colorado’s amended importation plan cuts prospective drugs by 78%. FDA Commissioner Califf warned about counterfeit medicines.
On February 27, Colorado submitted a revision of its December 5, 2022 Canadian drug importation proposal to the FDA. The amended application added Ozempic as an importation prospect and cut its drug list from 112 to 24 products. Read the new submission and PSM’s analysis.
Food and Drug Administration (FDA) Commissioner Robert Califf spoke about the dangers of counterfeit medicines, including a rash of fake and compounded versions of diabetes and weight loss drugs sold online. Watch the entire interview at Reuters.
Following eBay’s $59 million settlement with the Department of Justice, the Drug Enforcement Administration issued a letter informing roughly 450 e-commerce companies that they, too, must comply with federal reporting regulations over the sale of pill presses on their platforms.
Brassica Pharma Pvt. Ltd. has recalled eye ointment that may not be sterile. The recalled products were distributed by AACE Pharmaceuticals Inc and carry the AACE Pharmaceuticals, Equate and CVS Health brand names. Learn more about the recall at FDA’s website.
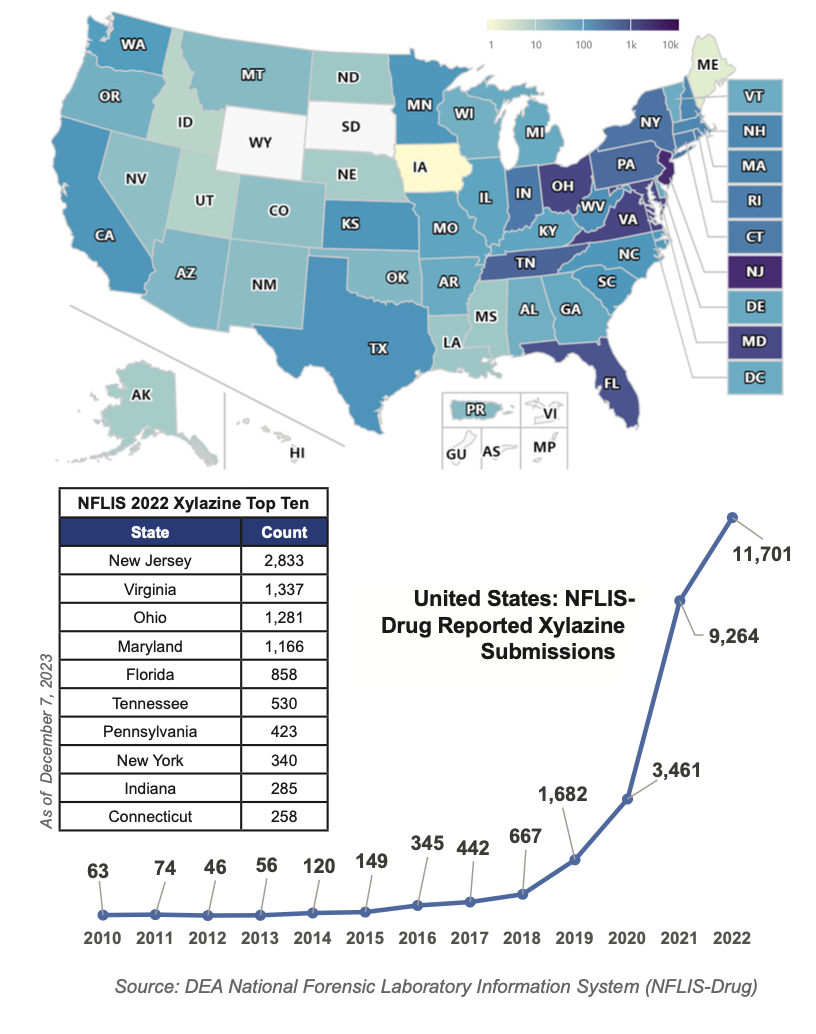
The Drug Enforcement Administration released a State and Territory Report on Enduring and Emerging Threats in January 2024. Consult it for a 2-page status update about U.S. drug trends.
Domestic News
The U.S. is prosecuting a couple allegedly responsible for Illegally imported medicines reaching cancer and rheumatology patients in California and Arizona.
The U.S. asked the U.K. to extradite a British couple who allegedly smuggled non-FDA approved cancer and rheumatological treatments from India and Sri Lanka and supplied them to patients at clinics they owned in California and Arizona. Court filings claim that the defendants defrauded government and private insurance companies, did not inform patients they were being treated with unapproved drugs, and violated additional safety practices by injecting patients with expired drugs and with ‘leftover’ medicine from single use vials.
Bronx, New York resident Whalesca Castillo received a four-to-eight-year prison sentence for second degree manslaughter for injecting 43-year-old Lesbia Ayala with body contouring silicone that killed her in 2017. Castillo was charged with similar offenses in 2011 and 2019. Injectable silicone is not FDA approved for any aesthetic procedure, and can lead to long-term pain, infections, permanent disfigurement, embolism, stroke, and death.
A polydrug bust in Darlington County, South Carolina, yielded a variety of illicit substances and two pill presses.
International News
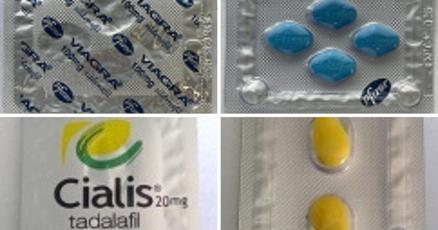
Fake Viagra and Cialis were seized from store shelves in Canada. COFEPRIS warned about unregulated and counterfeit medicines. Uzbek courts sentenced an executive over poisoned cough syrup.
Health Canada seized blister packs of counterfeit Viagra and Cialis from two Toronto convenience stores.
Mexico’s COFEPRIS issued warnings about sales of counterfeit and black market cancer and diabetes treatments, cosmetic injectables, eye drops, and more. Keep track of COFREPRIS’s alerts on their website.
Uzbek courts sentenced Singh Raghvendra Pratar, the executive director of a company that distributed poisonous cough medicines that killed 68 Uzbek children, to 20 years in prison.
The Nigerian Customs Service announced that it had seized 53 bags of counterfeit antibiotics between January 19 and February 29, 2024.
Authorities in Punjab, Pakistan banned specific lots of five essential medicines after uncovering a ring that sold counterfeit raw materials to legitimate pharmaceutical manufacturers.
In India, Mumbai’s Enforcement Directorate has charged more than six people with running a network of fake logistics companies, call centers, websites, consultancy firms, and pharmaceutical companies to traffick tramadol and other opioids into the U.S., the U.K. and Europe.
Indian authorities also seized counterfeit medicines in Telangana and Uttarakhand.