Problems inspectors found in compounding facilities
There are two types of compounding pharmacies that help patients. 503A pharmacies provide bespoke medications to suit patients’ requirements if mass-produced medicine can’t meet them. 503B pharmacies, known as outsourcing facilities, can create large batches of medication specifically for healthcare facilities to use in-office. There are approximately 7,500 compounding pharmacies in the United States and the vast majority do not have safety issues. Most of these are regulated by state pharmacy boards, who also conduct regular inspections.
Although compounded drugs are not FDA-approved, the agency does broadly monitor the pharmacies that produce them. The FDA inspects 503A compounding pharmacies for general surveillance and when it has reason to believe there are problems with a facility. The agency also handles the registration and inspection of 503B outsourcing facilities. Below is a list of some of the issues FDA discovered during these kinds of inspections.
More on diabetes & weight-loss injectables
- Counterfeits and compounding
- Compounding lawsuits
- What it means that the shortage of weight loss drugs is almost over
More about compounded medicine
Download PSM's guides:
| Compounding harm |
| Weight-loss injectables |
Inspection Incident Reports
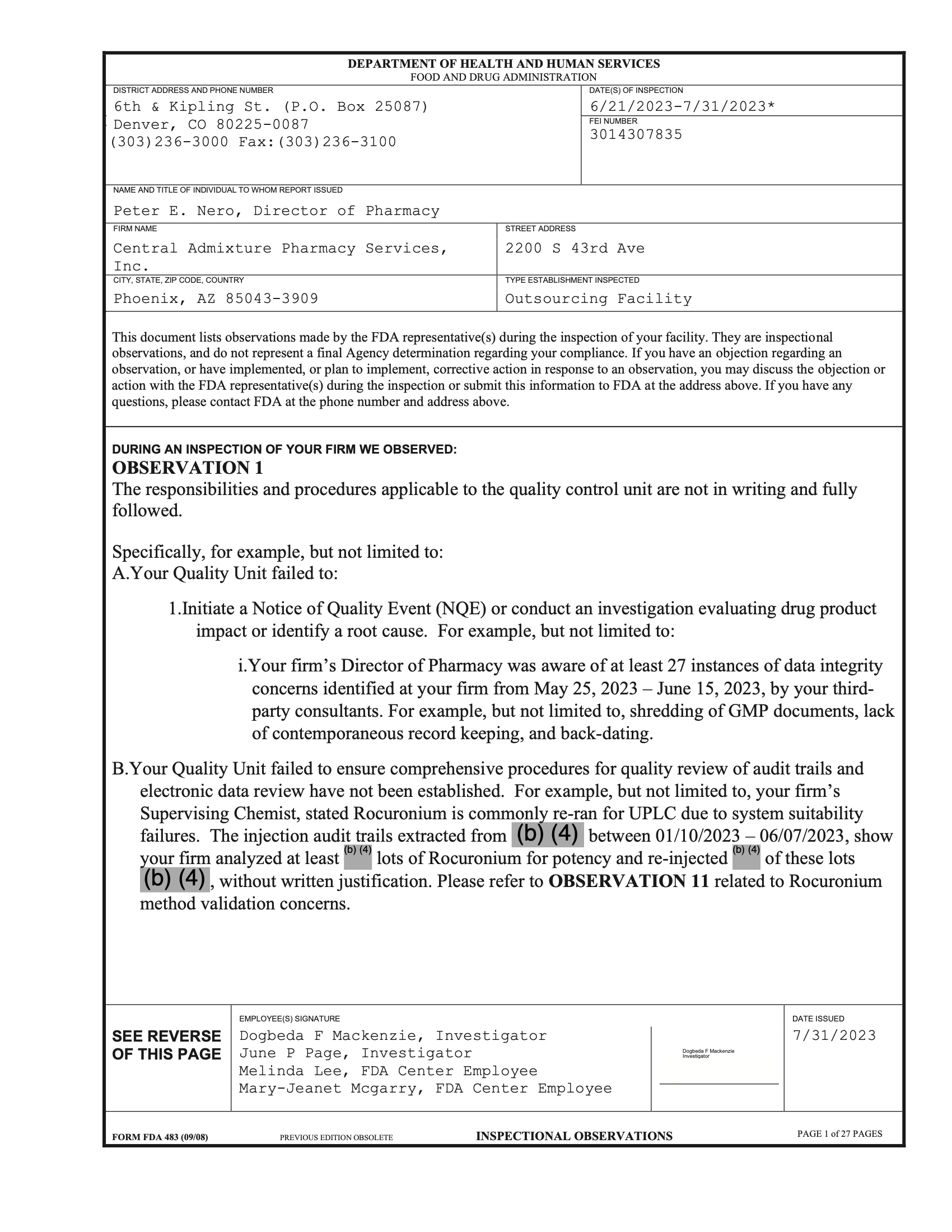
Report Date: 6/21/2023-7/31/2023
An IV technician at a facility in Phoenix, AZ, found a deceased lizard in a cleanroom where aseptic processing occurred. A review of the firm’s records also found the firm released drugs before verifying their quality.
Report Date: 7/30/2019-8/9/2019
An inspector observed that a facility in Davenport, FL produced “hazardous drugs without providing adequate cleaning of work surfaces and cleaning of personnel to prevent cross-contamination.” Additionally, “vermin were observed in [the] production area.” The facility had “visibly dirty equipment” and it released “drug product in which the strength differs from, or its purity or quality falls below, that which it purports or is represented to possess.”
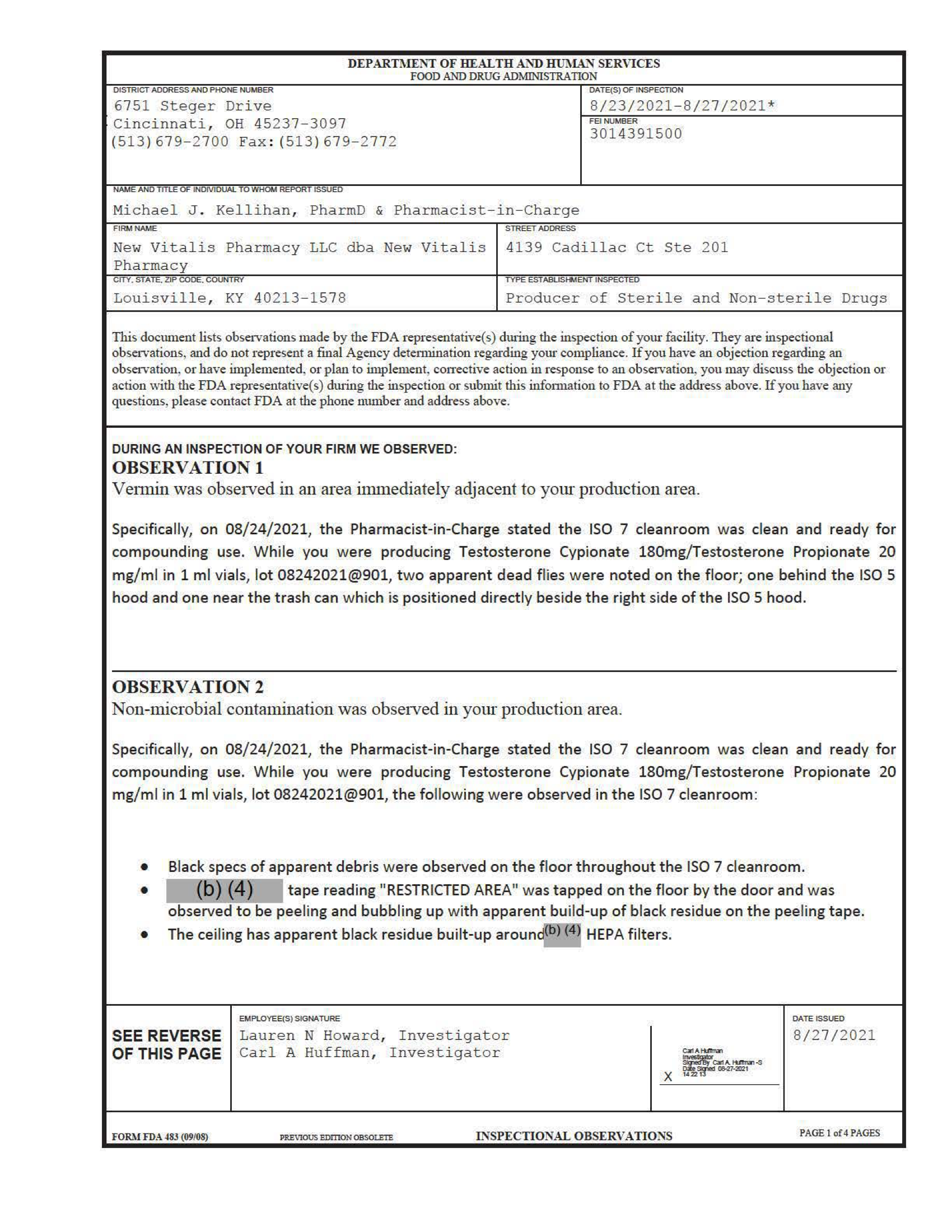
Report Date: 8/23/2021-8/27/2021
An inspector observing a facility in Louisville, KY saw vermin “in an area immediately adjacent to [the] production area ... Non-microbial contamination was observed in [the] production area.” The facility “produced hazardous drugs without providing adequate cleaning of utensils to prevent cross-contamination … Specifically, you have inadequate cleaning for your non-dedicated [redacted] blender, which blends active pharmaceutical ingredients such as progesterone and melatonin.”
Report Date: 10/13/2020-10/29/2020
Inspectors in Indianapolis, IN noted that “final containers/closures used for drug product intended to be sterile were not sterilized” and that an “employee produced a sterile drug product using expired material.” That “product was administered to a patient.” “Hazardous drugs were produced without providing adequate containment … to prevent cross-contamination.”
Report Date: 10/16/2023-12/1/2023
In Houston, TX a “compounding technician was observed using [their] sterile gloves to push down trash inside garbage bin … [and] failed to change sterile gloves before returning to aseptic filling operations.” The observer noted “use of ingredients not intended for pharmaceutical use in sterile drug production … bearing the text ‘Not for Use as Drug/API, or Drug Product.’” Inspector stated, “Your firm used the ‘Not for Use as Drug/API or Drug Product’ to compound…sterile drug product.” The inspector found products that had been dispensed with Rx which had been made with “Not for Use as Drug/API, or Drug Product” ingredients.
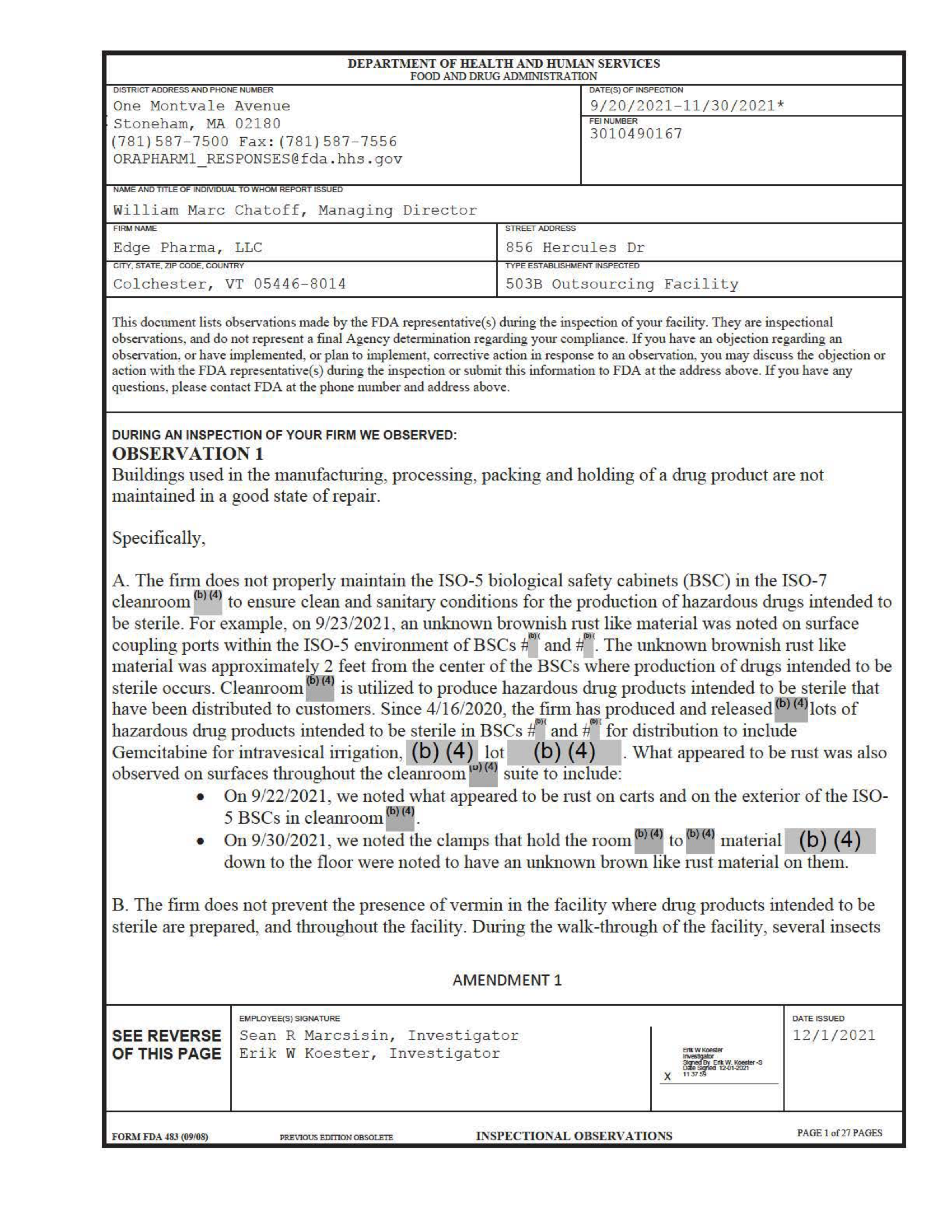
Report Date: 9/20/2021-11/30/2021
A facility in Colchester, VT was producing IV antibiotics and chemotherapies in cleanrooms that contained brownish rust material and green fuzzy substances. The inspector wrote, “Since 4/16/2020, the firm has produced and released [redacted] lots of hazardous drug products intended to be sterile in BSCs [redacted] for distribution to include Gemcitabine for intravesical irrigation.” He also said, “The firm does not prevent the presence of vermin in the facility where drug products intended to be sterile are prepared, and throughout the facility.” “Mold was recovered from an operator's fingertips after production of Vancomycin for IV injection.”