Have patients been harmed by unsafe drug compounding?
Compounded medicine is an important part of our healthcare system. It’s meant to be an exception to the normal drug supply chain, helping patients who cannot take a drug in its existing form or filling in when there are shortages of a life-saving medication.
However, compounding pharmacies are not regulated the same way as pharmaceutical manufacturing facilities, and that comes with risks to patients. Compounded medicines are not FDA-approved and the agency has "investigated many cases of serious patient injury linked to poor quality compounded drugs." In the past, compounding pharmacies have
- distributed medicine contaminated with dangerous bacteria because of non-sterile working conditions;
- used unapproved ingredients that have not been safety-tested for human use; and
- used cheaper, research-grade ingredients that are not pure enough for human pharmaceuticals.
More on diabetes & weight-loss injectables
- Counterfeits and compounding
- Compounding lawsuits
- What it means that the shortage of weight loss drugs is almost over
More about compounded medicine
Download PSM's guides:
| Compounding harm |
| Weight-loss injectables |
Case study: New England Compounding Center, 2012
In September 2012, the Center for Disease Control and Prevention (CDC), FDA, and local health departments across the U.S. began to investigate a multi-state outbreak of fungal meningitis and other infections among patients who had received contaminated steroid injections from New England Compounding Center (NECC) of Framingham, Massachusetts.
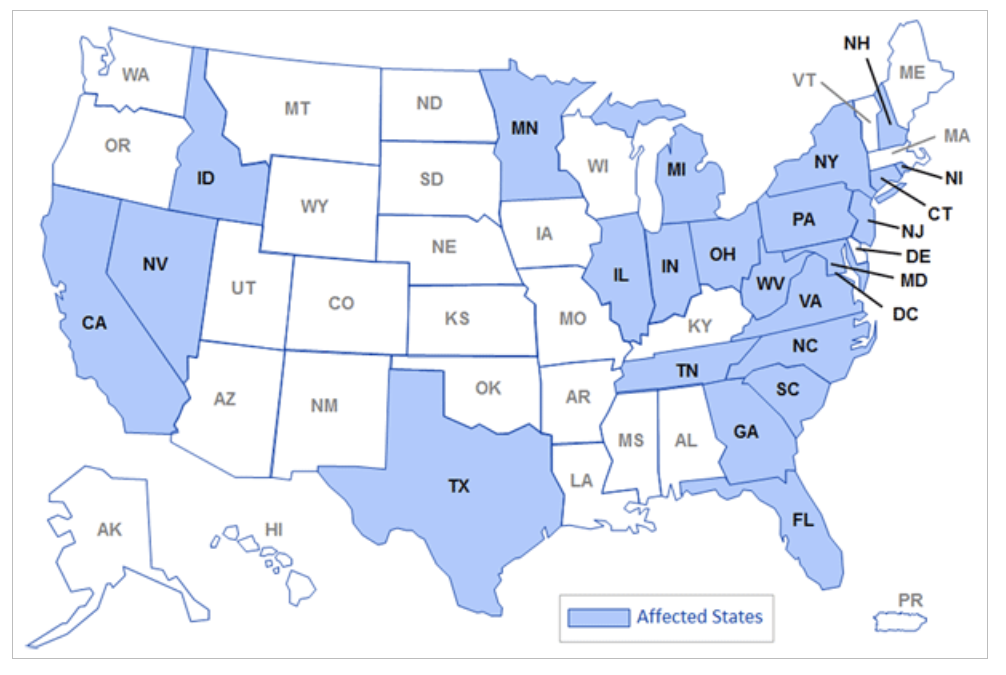
States that received NECC's contaminated injections in 2012 (CDC)
NECC was licensed as a compounding pharmacy, which allowed it to make medication for individual patients. Investigators discovered that NECC was functioning as a manufacturer, producing a broad range of drugs in large quantities. NECC distributed doses of the contaminated steroid injections to 75 medical facilities in 23 states.
The CDC traced the outbreak to three lots of methylprednisolone, a drug used for epidural steroid injections. Investigators found the same fungus in unopened vials and the cerebrospinal fluid of several patients, confirming that the fungus was the cause of the outbreak.
This incident was one of the largest public health crises caused by a contaminated drug as well as one of the largest documented fungal meningitis outbreaks in U.S. history. Regulators contacted over 13,500 people about their potential exposure. In 2012, 753 individuals in 20 states were diagnosed with fungal meningitis caused by the contaminated injections. Over 100 people died.
Other examples of dangerous compounding errors
Florida, 2019:
Forty-six patients suffered from eye inflammation, eye infections, high eye pressure, and floaters after receiving non-sterile eye injections made by an outsourcing facility.
Texas, 2017:
At least 43 cataract patients reported vision impairment, poor night vision, loss of color perception, light sensitivity, glare, halos, balance problems, and headaches after they received tainted post-surgery eye injections made by a pharmacy.
New Jersey, 2017:
Forty-one patients suffered septic arthritis from joint injections for osteoarthritic knee pain. An investigation found multiple breaches of recommended infection prevention practices during the preparation and administration of pharmacy bulk packaged products in an outpatient facility.
New York, 2016:
Seventeen oncology patients contracted fungal bloodstream infections and two died after receiving tainted compounded IV medications administered at an oncology clinic. Patients tested positive for E. dermatitidis, Rhodotorula mucilaginosa, or both fungi.
Texas, 2013:
Fifteen patients experienced bloodstream infections and two of them died after receiving contaminated calcium infusions made by a compounding pharmacy. Most infections were caused by Rhodococcus equi, a bacterium that lives in the soil and causes pneumonia in young horses.
Tennessee, 2013:
Twenty-six patients suffered from bacterial and fungal infections in the skin and soft tissue after receiving contaminated steroid injections made by a compounding pharmacy.
Illinois, 2012 - 2013:
An outbreak of Pantoea agglomerans bloodstream infections in 12 Illinois oncology patients came from compounded medication prepared near a contaminated pharmacy sink.
Florida, 2011 - 2012:
Forty-seven patients in nine states contracted fungal endophthalmitis from contaminated retinal drops or eye injections compounded by a pharmacy. Of the 40 patients for whom data was available, 39 reported lost vision.
Florida, 2011:
A dozen individuals in South Florida developed streptococcus endophthalmitis infections after eye injections of bevacizumab prepared by a compounding pharmacy. Three patients required the removal of their eyes.
Alabama, 2011:
Nineteen patients in six hospitals in central Alabama contracted Serratia marcescens bacteremia from intravenous feeding solutions. Nine patients died. An investigation revealed that a compounding pharmacy had failed to adhere to proper sterilization procedures.
See the FDA's page, Compounding: Inspections, recalls, and other actions to see other examples of compounding facility inspections.

