Patients face safety risks with counterfeit and compounded prescription weight-loss drugs
Officials warn of counterfeit and compounding pharmacy concerns as demand for weight-loss medicines surges.
Demand is skyrocketing for FDA-approved medicines that provide incredible benefits to patients living with diabetes and obesity. The injectables – GLP-1s like semaglutide and incretins that are commonly known as weight-loss drugs – have thus become a target for counterfeiters looking to make a profit. Bad actors have entered the market with perfect but dangerous lookalike counterfeits, and have also capitalized on the shortage with unsafe, fake, and compounded versions.
Because many of these medicines are on the FDA’s shortage list, compounding pharmacies are allowed to make alternative versions to address patients’ needs. But not all compounders and sellers are doing so legally. Experts predict that demand for these medicines and illegalities in compounding will continue to surge. Patients must understand the risks of not receiving a prescription from qualified providers and not using FDA-approved, manufacturer prescription drugs.
More on diabetes & weight-loss injectables
- Counterfeits and compounding
- Compounding lawsuits
- What it means that the shortage of weight loss drugs is almost over
More about compounded medicine
Download PSM's guides:
| Compounding harm |
| Weight-loss injectables |
Compounding FAQ
- What is compounding? Licensed pharmacists or facilities combine, mix, or alter ingredients of a drug to create a therapy tailored to individual patient needs.
- Why compound? This practice can help meet unique dosage requirements, alleviate allergy concerns, and provide a liquid alternative for those who cannot swallow pills.
- Who’s behind compounding? State boards regulate most compounding pharmacies (known as 503a), but a few federally regulated compounding pharmacies (503b) can make larger batches to help meet demand.
- Are compound drugs an equal alternative? No. The FDA does not review compounded medicines for its gold-standard safety, effectiveness, or quality
- Why is poor compounding hard to spot? There are no visual ways to tell whether a compounded injectable medicine has too much, too little, or no active ingredient.
- Have compounds harmed patients? Yes. The FDA recently issued warnings about compounders creating diabetes and weight loss injectables with impermissible starting ingredients. Sterility remains a challenge in compounding. Patients have been seriously injured and killed because of tainted versions.
Concerns about compounding
While compounders meet important medical needs, the operations can also present risks to patients. Most of the 7,500 compounding pharmacies in the U.S. are not as carefully monitored as FDA-inspected drug manufacturers. Sometimes, compounders use ingredients the FDA has not approved.
Compounded drugs are also vulnerable to counterfeiting because they do not receive serial numbers for inclusion in the U.S. track and trace system. This makes it more difficult to verify the safety and history of compounded products. The Partnership for Safe Medicines is concerned that not all state regulators have the resources to monitor every company participating in the explosion of compounded weight-loss injectables.
The FDA is concerned that compounders may be using non-FDA-approved ingredients like semaglutide sodium to make compounded injectables. (Found online July 11, 2024)
Sounding alarms on safety
- The FDA has issued myriad public warnings about compounded semaglutide and misbranded, unapproved counterfeit versions.
- Authorities shut down 250 sites selling the products after receiving reports of adverse events and unapproved ingredients.
- The Partnership for Safe Medicines has documented sales of weight-loss injectables on Etsy, the popular e-commerce platform. Etsy is not a licensed pharmacy, and neither was the source, which did not require the buyer to provide a prescription before purchase.
- In 2012 the New England Compounding Center produced three lots of injectables contaminated by insects and mold that sickened nearly 800 people and resulted in more than 100 deaths.
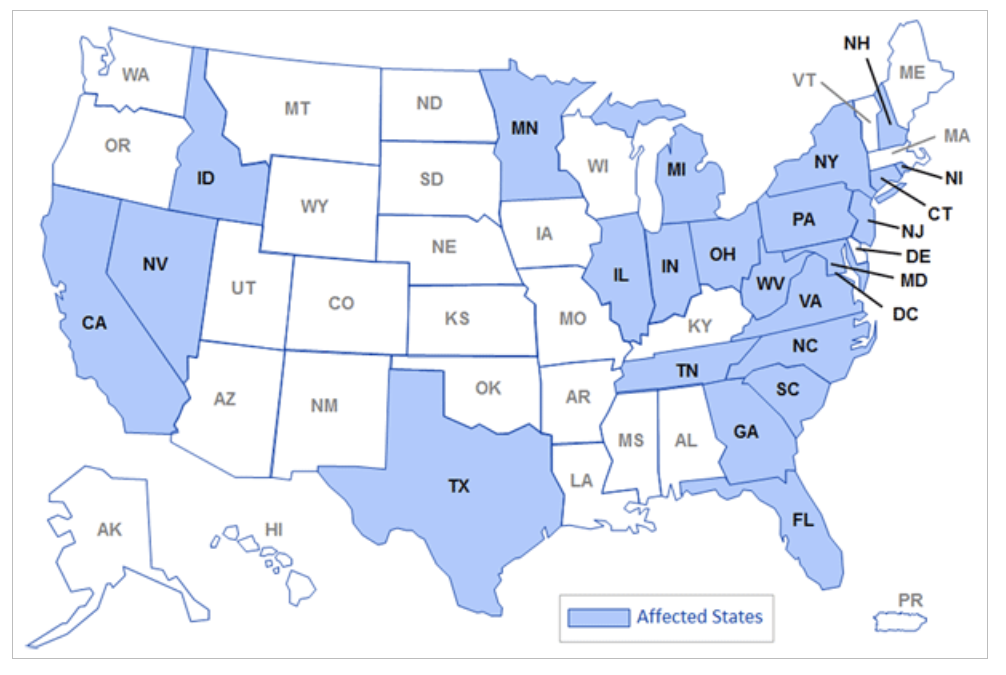
This CDC map shows where NECC shipped contaminated steroid injections in 2012.
Protecting patients
- Counterfeit weight loss injectables look realistic enough to fool consumers and may have deadly consequences.
- Products from foreign online pharmacies, med spas, weight loss clinics, and telehealth partnerships may not meet the FDA’s gold standard for safety and efficacy.
- Providers outside of regulated medical settings can sell compounded injectables that may come from non-sterile manufacturers or vendors entirely outside the U.S. inspection system.
- With no serial number system, determining the origin of compounded medicines and detecting counterfeits is extremely difficult.

Talk with your medical professionals about how to access safe, regulated injections if you are prescribed weight-loss medicines.