September 23, 2024: Administrative complaint filed alleging insulin price inflation by pharmacy benefit managers
Major Stories
The FTC filed an administrative complaint against PBMs alleging abuse. Owner of a compounding pharmacy discusses major recall.
The U.S. Federal Trade Commission filed an administrative complaint against the three largest pharmacy benefits managers alleging that the companies abused their economic power to inflate the price of insulin for U.S. patients.
The New York Post reported that a 503A compounding/mail-order pharmacy, Revive Rx Pharmacy, in Houston, Texas, whose owner is a repeat guest on the “The Joe Rogan Experience,” initiated a recall for some of its compounded products. According to the U.S. Food and Drug Administration’s (FDA) enforcement report, in April, Revive Rx recalled 751 10mg/0.5mL vials of tirzepatide, the active pharmaceutical ingredient in Zepbound, a weight loss drug. Instead of tirzepatide, the vials contained testosterone.

The FTC says PBMs are driving up drug costs. Learn how.
Domestic News
Counterfeit pharmaceutical seizure statistics from fiscal year 2023. Overdose deaths look to be trending downwards.
Data compiled by the Centers for Disease Control and Prevention show that overdose deaths are down by roughly 10.6% nationally, with some areas reporting decreases of over 30%.
In FY 2023, U.S. Customs and Border Protection seized 3,037,098 counterfeit health and safety items. Nearly half of those items - 47.9% - were counterfeit pharmaceuticals.
A lawsuit was filed by the family of a man in New Jersey who died after ingesting Neptune’s Fix Elixir. Neptune’s Fix contains tianeptine, an antidepressant drug not approved in the U.S. and that the FDA warns can cause adverse reactions, including seizures, coma, and death.
A Tulsa, OK man, who was found in possession of a pill press and counterfeit pills, received a federal prison sentence of 181 months.
Police in Zachary, LA seized a pill press and an array of drugs while executing a search warrant.
The Biden-Harris Administration took new actions to protect American consumers, workers, and businesses by cracking down on foreign companies that are abusing de minimis shipment regulations to send unsafe and unfairly traded products into the U.S
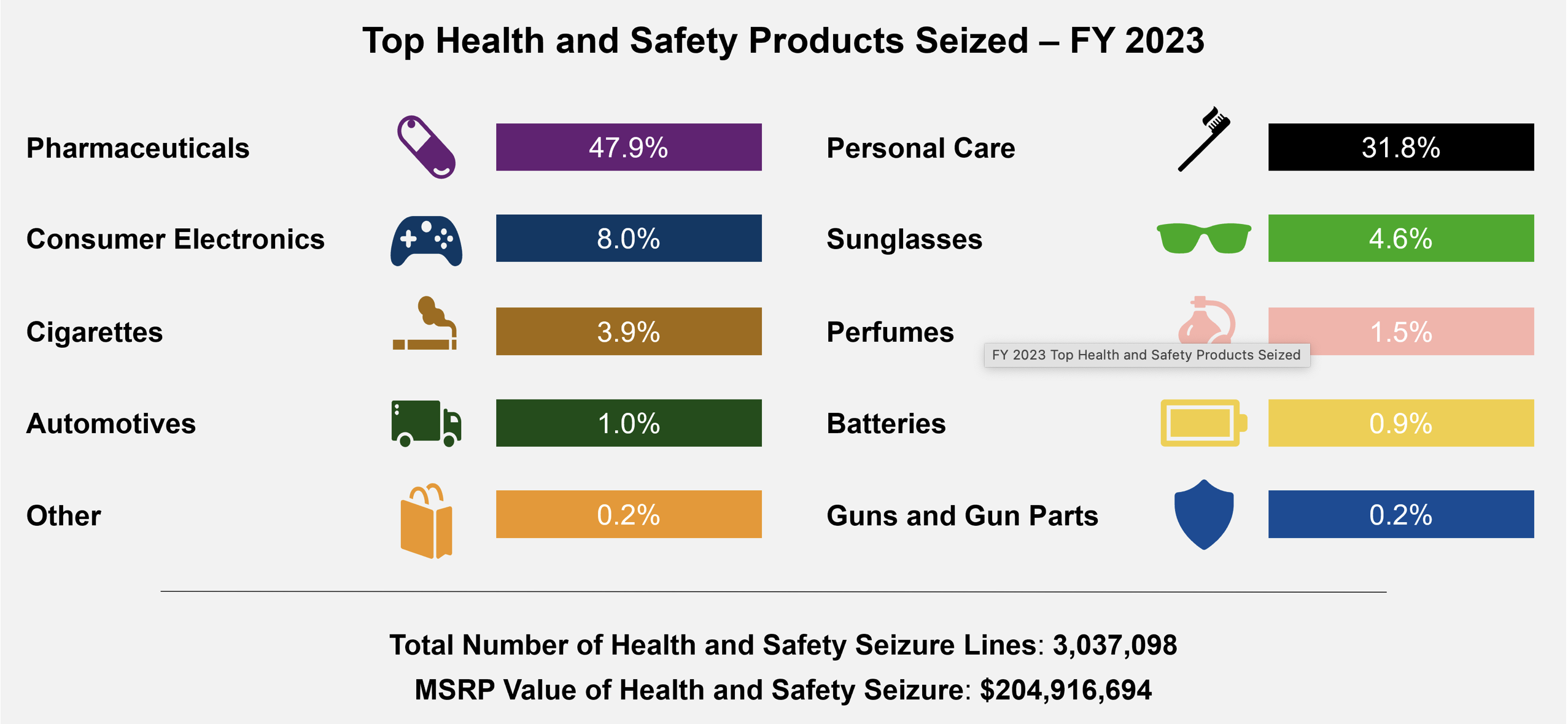
Counterfeit pharmaceuticals represented nearly 50% of the items seized by U.S. Customs and Border Protection in fiscal year 2023. Source: CBP
International News
Multiple updates from Mexico’s health authority and counterfeit medicines and medical devices destroyed in Nigeria.
Since the beginning of June, Mexico’s health authority COFEPRIS has issued 20 alerts. Seven of the alerts - Keytruda, Mabthera, Pomalyst, L-Aspal-P, Eutirox, Ramiven, and Phoxelon 500 - were for medicines used in the treatment for various cancers. Additionally, these COFEPRIS alerts included multiple instances when unapproved medications were being found for sale either online or in unauthorized locations.
Nigeria’s National Agency for Food and Drug Administration and Control seized and destroyed substandard products, including counterfeit medicines and medical devices, worth N43 billion (over $26 million).
