September 30, 2024: 27 individuals indicted in Puerto Rico over wholesale distribution of misbranded and diverted medicine
Major Stories
HHS and FDA brought charges against 27 individuals and the Treasury issued additional sanctions.
An investigation in Puerto Rico led to a total of 27 individuals being indicted for their alleged involvement in the unlicensed wholesale distribution of sale of misbranded and diverted medications to pharmacies. The defendants included unlicensed wholesale distributors and pharmacy owners and employees who, in violation of the Federal Food, Drug, and Cosmetic Act (FDCA) and the DSCSA, conspired to sell misbranded and diverted prescription drugs from unauthorized trading partners to pharmacy customers, without the customers’ knowledge that the products were misbranded and diverted.
The U.S. Treasury imposed new sanctions against companies in Mexico owned by designated Sinaloa Cartel fentanyl traffickers, including a retail pharmacy/supermarket in Nogales that is owned, controlled, or directed by a known drug trafficker.

A picture of Farmacia y Mini Super Trinidad, the pharmacy/supermarket sanctioned by the U.S. Treasury Department. Source: Google
Domestic News
CBP in Texas and Ohio seized medication, major counterfeit pill manufacturing ring busted in Connecticut, and warning letter.
Agents with U.S. Customs and Border Protection (CBP) seized 280 boxes of undeclared medication from a vehicle attempting to enter the U.S. from Mexico at the port of entry in Presidio, Texas.
A week-long joint operation between CBP and U.S. Food and Drug Administration (FDA) in Cincinnati, Ohio led to the seizure of nearly $270,000 worth of non-FDA-approved medications being sent to the U.S. from China, South Korea, India, Italy, Guatemala, United Kingdom, and Canada.
A federal investigation disrupted a counterfeit pill ring in Connecticut that was allegedly manufacturing and shipping a variety of fake pills to customers across the U.S. who ordered the pills via the dark web.
Police in Monroe, Louisiana seized a pill press capable of producing 5,000 pills in one hour.
The FDA issued a warning letter to nomida.biz on September 12, 2024. The FDA’s review suggests that the company could be introducing unapproved and misbranded semaglutide and tirzepatide drug products into interstate commerce.
An article in Pharmaceutical Technology offers an excellent recap on how the inclusion of certain weight loss drugs on the FDA’s drug shortage list has led to some compounding pharmacies acting in ways that have raised patient safety concerns.
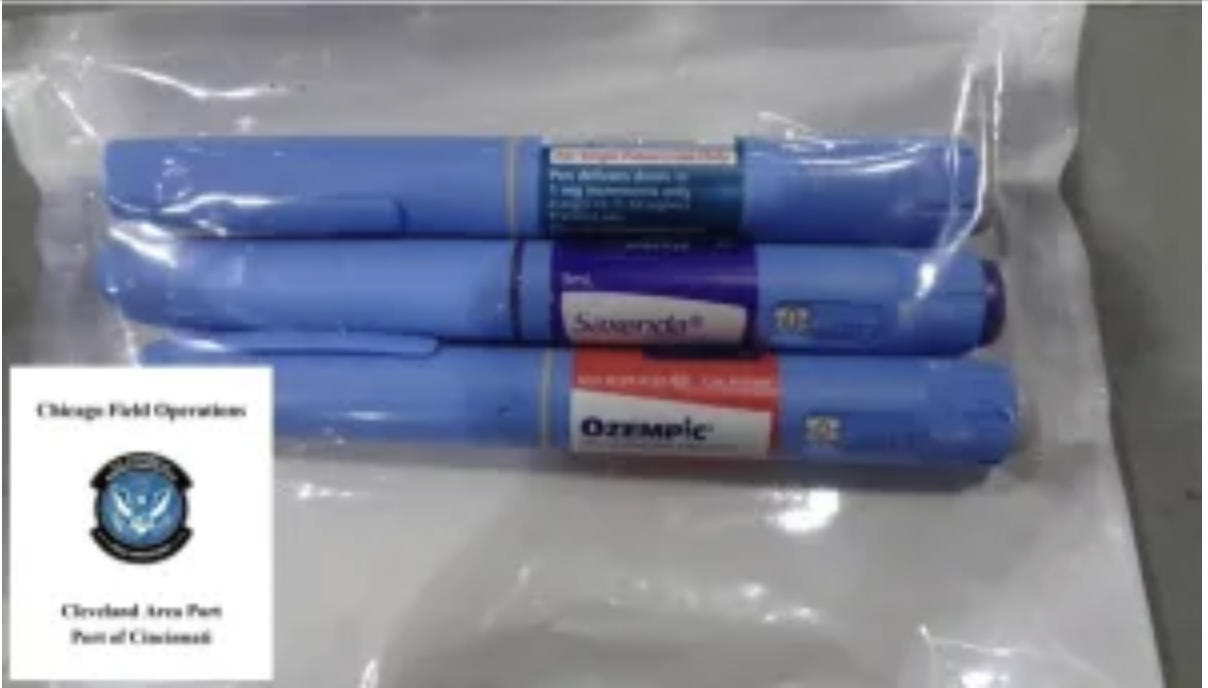
Non-FDA-approved weight loss injections seized during the CBP-FDA week-long joint operation in Ohio. Source: CBP
International News
1,000 boxes of a counterfeit arthritis drug seized, government run hospitals in Maharashtra received counterfeit antibiotics, and fake cancer medication seized in India.
After searching a pharmacy and a home in Gurugram Sector 39, police arrested three individuals and seized 1,000 boxes of counterfeit Tofajak, a medicine used to treat rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, and ulcerative colitis.
More than half a dozen people have been charged in India for allegedly manufacturing and selling counterfeit antibiotics to government run hospitals in the Indian state of Maharashtra. The antibiotics contained talcum powder and starch.
Pimpri Police in Maharashtra seized counterfeit Adcetris injections, a cancer medication, and arrested the shopkeeper for his alleged involvement in the manufacturing and sale of counterfeit medicine. Adcetris has not been approved yet for use by patients in India.
Veterinarians in Singapore are warning pet owners about counterfeit pet medication being sold online.
