Prescription Drug Affordability Board Activity through December 18, 2024
Activities Summary
Colorado: The Prescription Drug Affordability Board (PDAB) met on December 6, 2024 to hear and discuss public comment before proposing and approving revisions to policy and procedures around affordability reviews. The PDAB updated the state's Prescription Drug Affordability Review Board and Advisory Council (PDAAC) on December 12, 2024.
Maryland: On November 25, 2024 the PDAB reviewed public feedback on its draft policies for upper payment limits, proposed and approved policy changes, and approved its annual report to the legislature. The board chose six medicines to undergo a cost review: Farziga, Jardiance, Ozempic, Trulicity, Dupixent and Skyrizi. Maryland's Prescription Drug Affordability Board Stakeholder Council (PDASC) met on December 16th to discuss clarifications to regulations. The definition of upper payment limit has been adjusted to be in terms of a “system net ingredient cost” that combines the patient's out-of-pocket cost plus and the payer is paying.
Oregon: At its November 20, 2024 meeting, the PDAB heard testimony regarding the Upper Payment Limit Report, made revisions, and approved it to send to the legislature. A subsequent meeting on December 18th devoted most of its time to potential changes to Oregon Senate Bill 844, which established the state's PDAB.
Washington: On November 13, 2024 the state's PDAB approved its Eligible Prescription Drugs Policy, delved into drug selection criteria and affordability reviews. and heard public comment. A December 10th meeting of the PDAB Advisory Group also focused on the drug selection process.
Activity by State
Colorado PDAB meeting, December 6, 2024
Meeting materials | Presentation | Recording
After the call to order, roll call, and member updates the board approved minutes from the previous meeting.
Director Updates by Lila Cummings and Board Business
Cummings provided an overview of the meeting agenda.
Discussion on Revisions to PDAB Policy and Procedures
Executive Session
The board met in executive session to receive legal advice regarding use of quality-adjusted life years (QALYs) as part of affordability reviews pursuant to section 24-6-402(3)(a)(II), C.R.S.
Rulemaking Hearing
Prescription Drug Affordability Board Rule 3 CCR 702-9: Part 1 - General Provisions and Part 3 - Affordability Reviews of Prescription Drugs
Board Deliberation
The board voted on and approved updates to Parts 1 and 3 of the Affordability Review rule as amended.
PDAAC Appointments and Reappointments
The board voted unanimously to reappoint Marc Reese, PBM representative; R. Brett McQueen, representing organizations that research prescription drugs and Katelin Lucariello, representing name brand drug manufacturers for a second 3-year term.
The board has open positions on the PDAAC for statewide healthcare advocacy organizations, labor unions, carriers, health care professional with prescribing authority, manufacturers of generic drugs and pharmacists. No one from labor unions or carriers has applied. The board unanimously voted to appoint Bob Mulch from AARP Colorado, Richard Miranda from St. Joseph Hospital, Fayez Azeez, CEO of Saptalis Pharmaceuticals, and Ingrid Pan, pediatric rheumatology pharmacist, to the PDAAC for a 3-year term.
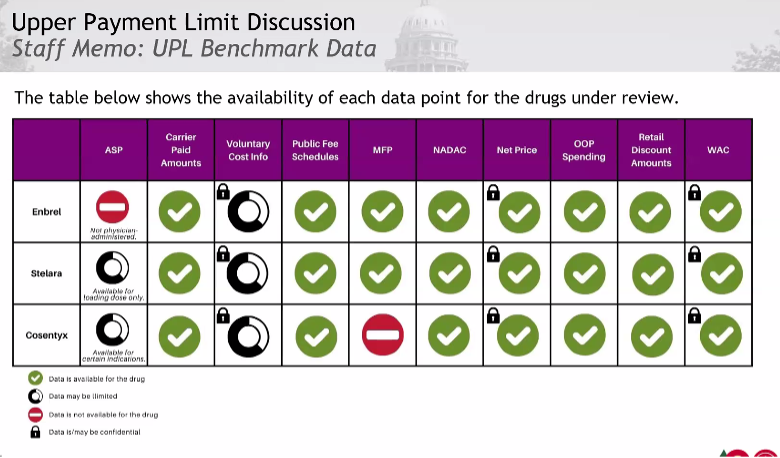
Upper Payment Limits Rulemaking Structure
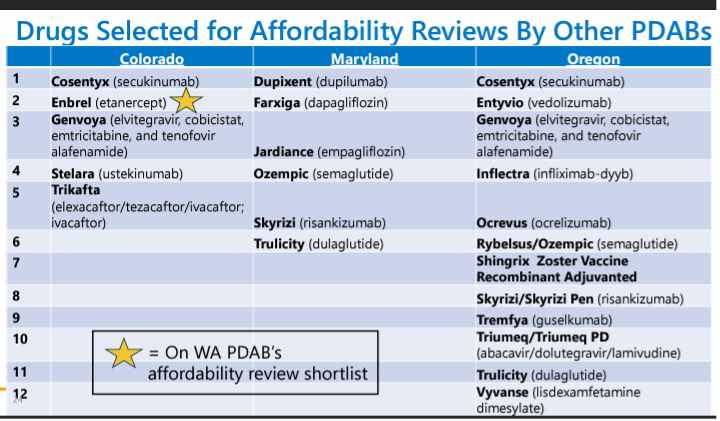
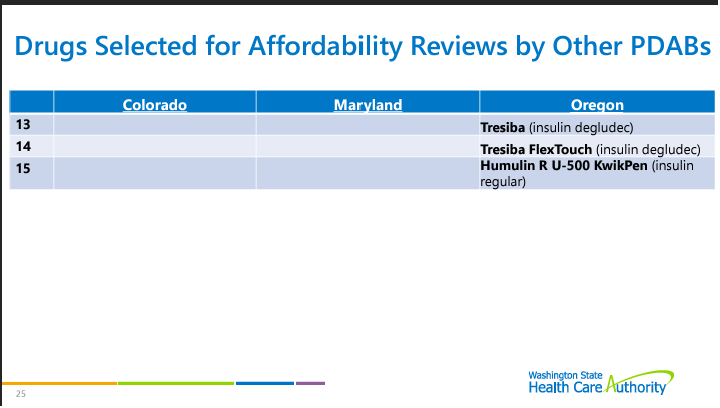
The staff recommended starting with Enbrel (etanercept). There will be three rulemaking hearings. Staff will present data for UPL benchmarks for the drug and its therapeutic alternatives and stakeholders will provide testimony at the hearings, with the rulemaking concluding at the third unless the board decides otherwise.
Upcoming meetings
PDAB Meeting: January 17 at 10 am MT
Colorado PDAAC meeting, December 12, 2024
PDAB Report Out
The board will not be using multi-criteria decision analysis for this round of appeals but may want to use it in future rounds. PDAAC questions were passed on to the PDAB board.
PDAAC Introduction
PDAB history was reviewed for new members. New members of the PDAAC were introduced to existing members.
Executive Session
Legal training for new PDAAC members in closed session.
Director Updates & PDAAC Business
Debrief of September 6 Joint Meeting
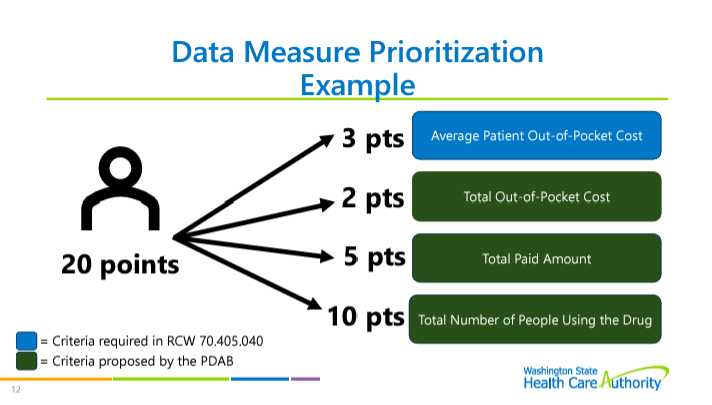
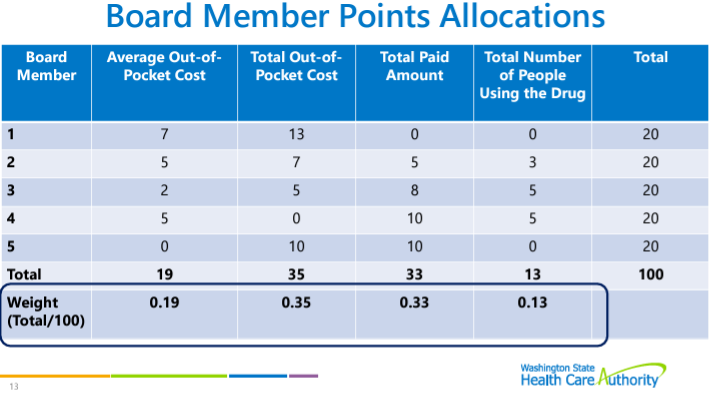
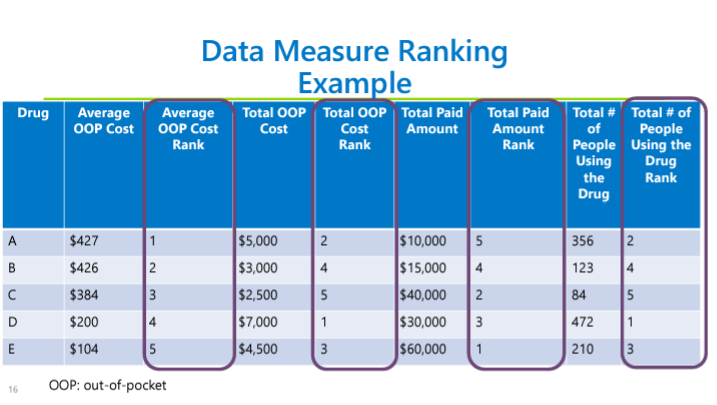
Lisa Cummings reviewed the upper payment limit process and rulemaking structure. Multi-criteria decision analysis (MCDA) will not be used by the PDAB for this round of upper payment limits (UPLs), but the board may use MCDA in 2025. Cummings reminded the PDAAC that the board is looking at a UPL for Embrel first, with at least three rulemaking hearings. The data submission guide structure was reviewed.
Board asked questions about timing and input for UPL analysis.
Upper Payment Limit
Staff Memo to the PDAAC
The PDAAC uses the staff memorandum as a guide for conversation and to help the board with input on the UPLs, focusing on impact of the UPL on patients and supply chain entities. PCAAC members asked questions about the UPL process.
PDAAC 2025 Meeting Schedule and Discussion Topics
Meetings will occur every other month through 2025.
Public Comments
No oral public comments were given.
Written comment was provided by Tiffany Westrich-Robertson, Ensuring Access through Collaborative Health (EACH) Coalition.
The next PDAB meeting will be January 17, 2025.
Maryland PDAB meeting, November 25, 2024
Opportunity for Public Comment
Harry Gewanter, board member of Let My Doctors Decide submitted written comment, noting that Maryland’s proposed implementation of UPL regulations may restrict access to necessary medications for patients, particularly those with rare or complex conditions. As written, the draft regulations could limit stakeholder participation and give the Board significant control over inputs, excluding certain viewpoints. Physicians and patients are concerned that the current approach risks overlooking individual patient needs and could disadvantage certain populations. Recommendations should include the roles of all players in the system, focusing on what patients pay.
Draft Regulations
The executive director reviewed the progress and next steps for UPL regulations. The draft upper payment limit action plan was presented to the board and was approved by the legislative policy committee on October 22, 2024. The next step is to adopt regulations that establish the policy review process. The draft includes an amendment to definitions, new regulations for hearings procedures, and a new chapter for the policy review final action and upper payment limits.
The draft regulations were posted on October 28, 2024. Public comment was received until November 8.
The board seeks emergency action on proposed amendments to .05 under COMAR 14.01.04 Cost Review Study Process. The text of the proposed amendments is available here.
Summary and Presentation by the Executive Director
Draft regulations were approved unanimously by the board.
Annual Report
On December 31, 2024, the board will submit an annual report to the Senate Finance Committee and the House Health and Government Operations Committee. It will review price trends for prescription drug products, the number of products reviewed by the board, and recommendations for further legislation to make those products more affordable.
The board discussed the cost review study process, referring eight drugs to the stakeholder council for input. Six drugs were selected for study, and a request for information was issued for four of them. The board also included a list of board actions related to the cost review process. Six drugs are subject to the cost review process, and no results are yet available. The board will summarize the results of each cost review study and work on the upper payment limits and the upper payment limit action plan in future reports. The drugs selected for the cost review study process are:
- Farziga
- Jardiance
- Ozempic
- Trulicity
- Dupixent
- Skyrizi
Biktarvy and Vyvanse were not selected.
The report cites the 2024 National Drug Pricing Trends report highlighting an increase in out-of-pocket costs per retail prescription, patient aggregate out-of-pocket cost, and list price. However, this growth is slower than in previous years. The report also notes an increase in the launch prices of new drugs in the 2023 market and mentions the need to reconcile the proportion of premiums going towards prescription drug products with overall healthcare spend. The board is looking into Maryland-specific data and may release future reports covering this.
By April 2024, 11 states established prescription drug affordability boards, with four granting them authority to establish upper payment limits. Many states and the federal government are working on legislation addressing affordability.
The 2022 IRA restricts drugmakers' price increases for Medicare treatments. The program can negotiate prices for a limited number of existing drugs. The first cycle of Medicare price negotiations involved 10 drugs. Negotiated prices were revealed in 2024, and they will take effect in 2026. The IRA restricts Medicare enrollees' out-of-pocket spending to $2,000/year and insulin to $35/month.
The U.S. biosimilars market is growing, with estimated savings increasing by 30% from 2022 to 2023. Biosimilars only make up a third of the biological product market in 2023. In June 2024, the FDA issued a draft guidance to ease interchangeability requirements for manufacturers. However, many challenges remain to biosimilar uptake.
From 2019 to 2023, the number of non-diabetic patients starting GLP-1 RA (Glucagon-Like Peptide-1 Receptor Agonists) therapy in the U.S. climbed by over 700%. Off-label use of GLP-1 RAs nearly doubled from 2019-2023, leading to domestic and international shortages. Medicare does not cover weight loss drugs. Maryland Medicaid covers certain GLP-1 RAs for type 2 diabetes treatment. Maryland will investigate the impact of requiring Medicaid to cover weight loss GLP-1 RAs.
The Board will continue to collect data and analyze it in accordance with COMAR 14.01.04.05 for the Cost Review Study Process. They expect to make a preliminary determination on whether the drugs in the process may cause affordability issues in late 2024 or early 2025. They also plan to propose regulations establishing the Upper Payment Limit framework.
The board voted to approve the annual report.
Cost Review Study Process
The cost review study has received all expected data and is drafting documents for public release. The board is expected to conduct a preliminary affordability analysis in upcoming meetings.
- Data Discussion
- Farxiga - Over 60-day comment period, patient input and physician comments were received for Farxiga, and seven RFIs were responsive to data related to Farxiga.
- Jardiance - Jardiance received two comments, and seven responses from the RFIs, providing valuable information for a comprehensive summary.
Administrative Update
The cost review study process continues.
Chair’s Update and Adjournment
The chair expressed gratitude to the staff. Meetings will be virtual in January and March. The board adjourned until January 27th, 2025.
Maryland PDASC meeting, December 16, 2024
After call to order, roll call and November 4, 2024 meetings minutes approval, the PDASC provided opportunity for public comment. No public or written comments were given.
Upper Payment Limit Update – Presentation
Cost Review Study Process – Presentation
No presentation was shared. The PDASC was given an update by Dr. York.
The drugs under cost study review currently are Farxiga and Jardiance.
Data has not yet been published and is not available for the public. This should be happening through early 2025. The next two drugs are Ozempic and Trulicity. The board will review the information from the drugs called “the dossier.” For all diabetes drugs they included all drugs within similar therapeutic guidelines. They are prioritizing comparison across the SGLT2 (Sodium-glucose cotransporter-2) class.
Administrative and Co-Chair’s Update
The next meeting will convene on February 24, 2025.
Oregon PDAB meeting, November 20, 2024
The next PDAB meeting will be Wednesday, December 18, 2024, 9:30 A.M. Pacific Time. (Agenda)
The Oregon PDAB released a year-end in review newsletter, available here for review.
Oregon PDAB meeting, December 18, 2024
After call to order, roll call, declaration of conflict of interest and approval of November 20, 2024 minutes, the executive director, Ralph Magrish, gave a program update. The board has welcomed new members, including Chris Laman, Dan Kennedy, and Lauri Hoagland, and seated new chairs and vice chairs. The research and analytic team has been staffed up. The board expressed gratitude to board members, staff, contractors, and the counsel of the Oregon Department of Justice for their efforts.
The meeting was adjourned at 12:22.
Washington PDAB meeting, November 13, 2024
Washington PDAB Advisory Group Meeting, December 10, 2024
Agenda | Recording pending
After welcome and introductions, the board discussed the advisory group lead.
Drug Selection Process
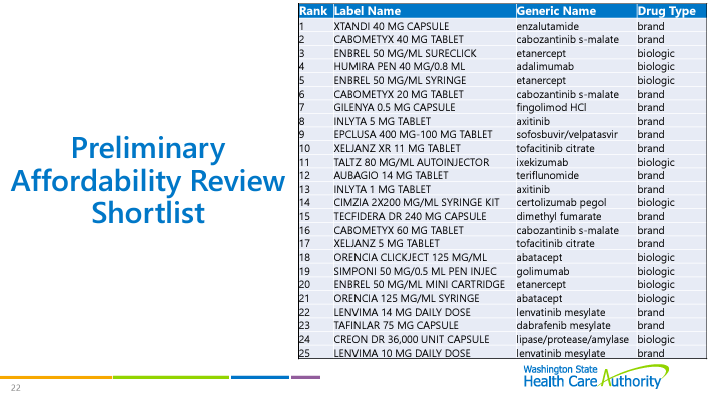
The board discussed the drug selection process. See previous meeting notes for details on the process. However, board members changed their votes between the meetings, which adjusted the rankings. The board has new lists of specialty and non-specialty drugs that are ranked for affordability review. The board will look at these two shortlists to pick drugs for affordability review.
The board is the process of building a website dashboard to allow the board to look at data points on drugs. Staff gave a demonstration on the use of the dashboard. The dashboard will be available to the public.
Discuss Drug Selection Recommendation Strategy
(Advisory group members each select five drugs to recommend to the board)
Advisory members engaged in discussion of potential missing information and duplication of drugs because of different dosages in the lists. Advisory board members were asked to submit questions and lists of suggested drugs for affordability reviews with reasonings.