Prescription Drug Affordability Board Activity through January 27, 2025
Activities Summary
Colorado: On January 17th, the PDAB reviewed Draft Upper Payment Limit Data Submission Guidance to discuss whether it was sufficient to begin an upper payment limit review on Enbrel at the March 7th meeting. A January 23rd meeting of Colorado's PDAAC involved reviewing the the upper payment rulemaking structure and soliciting feedback on the data submission guide.
Maryland: In a meeting on January 27th, Maryland's PDAB considered and approved amendments to the Cost Review Study Process. There was written and oral comment about the drugs Jardiance and Farxiga. The Board may have ad hoc meetings before its next scheduled meeting on March 24, 2025.
Oregon: Board members met on January 15th to undertake an annual review of policies and consider the preliminary list of prescription drugs and insulin for affordability review. The next meeting will be February 19, 2025.
Washington: At its January 15th meeting, Washington's PDAB voted to accept a drug selection policy and developed a short list of drugs that will face affordability reviews. The board also reviewed a data dashboard about eligible drugs. The next meeting will be March 19, 2025.
Activity by State
Colorado PDAB meeting, January 17, 2025
The Enbrel Upper Payment Limit rulemaking will begin on March 7, delayed from the January 17 meeting. The rulemaking guide, updated January 8, 2025, is available for review.
Director updates & board business
Revisions to PDAB Policy and Procedures:
Policy No. 4 (Affordability Review Policy and Procedure)
This discussion was tabled due to lack of majority of board members.
Draft Upper Payment Limit Data Submission Guidance
Draft Upper Payment Limit Data Submission Guidance
See document, draft 1/14/2025. The Executive Director Lila Cummings reviewed the document with the board. The purpose of this review was to discuss whether the submission guide is sufficient for use with the first drug subject to the UPL review, Enbrel (etanercept). The board discussed separating types of actors beyond the categories below, i.e. 340b entities vs. non-covered entities. They discussed how data should be parsed and whether the submission guide provided granular enough detail. They discussed reviewing this and asking for more information from the advisory council. Their goal is to finalize and publish the guidance by end of February. Staff will present data at the March 6 meeting. Stakeholders are asked to submit information by April 11.
- Submissions Regarding Impact to Older Adults (+65) and Persons with Disabilities
Stakeholders with lived experience or expertise on the impact of prescription drugs on older adults and persons with disabilities are encouraged to submit information to the Board for a drug's UPL rulemaking. This includes qualitative and quantitative analyses, effectiveness of the drug and its therapeutic alternatives, differences in effects for older adults or persons with disabilities, and any other information. The Board will not accept research using QALY or similar measures, so submitted information should be well cited and not contain QALYs or similar measures. - Submissions from Supply Chain Entities
The Board is seeking data on the potential impact of a UPL on various supply chain entities, including manufacturers, wholesalers, PBMs, carriers, and providers and pharmacists. The requested data includes transaction details, rebates and discounts, and plan design. The data should include current data for each category and projections on how the data may be impacted after a specific dollar amount UPL is set. The data should be provided at multiple years, describe data sources, show calculations, outline methodologies, and specify if data is presented at the NDC level, aggregate level, or another level. The Board is seeking information from the following entities:- Manufacturers
The Board is asking manufacturers to provide information on factors affecting their decision to sell or purchase a prescription drug and how a potential UPL would impact patients, specifically in terms of lower premiums or out-of-pocket amounts. Manufacturers are encouraged to report current and projected data at the NDC level for transactions, units, rebates, discounts, and assistance programs offered by the manufacturer. The data should include the total sales, average price charged, units sold, rebates and discounts, and the percentage of sales to 340B providers. - Wholesalers
Wholesalers are asked to provide current and projected data on sales, purchases, reimbursements, units and utilization in Colorado. The data should include the total sales, average price per unit, total units sold and purchased, and average rebates or discounts received from a manufacturer for business in Colorado. The Board is encouraged to understand the impact of a potential UPL on wholesalers and patients, particularly in terms of lower premiums or out-of-pocket amounts. - Carriers
The Board is asking carriers to provide information on factors affecting their decision to cover or reimburse a prescription drug and how a UPL might impact patients. This includes transactions, rebates and discounts, unit utilization, rebates and discounts, and plan design. Carriers are encouraged to report current and projected data at the NDC level for each type of plan, including net price, average revenues from rebates, and other retail discounts and fees. The data should also include utilization management practices and potential impacts on formulary placement, cost-sharing, benefit design, and copayment and coinsurance amounts. - Pharmacy Benefit Managers
The Board is seeking information on the impact of a potential UPL on PBMs, including factors affecting their decision to cover/reimburse prescription drugs and the potential impact on patients. PBMs are encouraged to report current and projected data on transactions, total reimbursement amount, gross and net revenues, patient cost-sharing, units and utilization. The net price of a drug in Colorado should be reported, along with the average revenues from rebates. The total discounts and fees paid to pharmacies, prescription drug networks, and pharmacy services administrative organizations should also be included. Utilization management practices, formulary placement, cost-sharing, benefit design, and copayment and coinsurance amounts should also be discussed. - Pharmacies
The Board is seeking information on the impact of a potential UPL on independent, specialty, or retail pharmacies. Factors affecting the decision to sell or purchase a prescription drug include the average purchase price and total sales price of the drug. The average price and reimbursement for the drug should include minimum, maximum, median, and mean. Units and utilization should include the total number of prescriptions filled and the number of patients that filled the prescriptions for the drug. Rebates and discounts, such as cards, coupons, manufacturer discounts, or other discounts, should be described and the total dollar amount or percentage of any discounts patients may receive for the drug.
- Manufacturers
Public comment
Written comments were provided by PhRMA, CANN and Act Now Colorado.
PhRMA: The Pharmaceutical Research and Manufacturers of America (PhRMA) has expressed concerns about the PDAB's draft memorandum, "Upper Payment Limit Benchmarks - Cost & Price Methods and Data Details," which lacks clear and meaningful standards regarding the implementation of the PDAB Statute. The draft memo outlines data points for each drug under review and identified therapeutic alternative but lacks sufficient methodology for assessing and weighing benchmarks. PhRMA requests the Board to revise the draft memo to establish clearer standards and consider the broader implications of a particular UPL on patient access, therapeutic alternatives, and supply chain stability.
The draft memo lacks clear date ranges for data benchmarks, which could lead to inconsistent comparisons. The draft also fails to provide adequate processes to verify the reliability of data used to support a potential UPL or to account for limitations in All-Payer Claims Database (APCD) data, such as the absence of diagnosis codes or Medicare Fee-for-Service data. PhRMA has concerns about the inclusion of the Maximum Fair Price (MFP) benchmark, which could disrupt Medicare Part D beneficiaries' access to medicines due to the Inflation Reduction Act. The draft also fails to account for intra-product differences impacting pricing and access.
The PDAB's draft memo lacks clear standards for identifying and assessing therapeutic alternatives, which could lead to inaccurate UPLs. PhRMA recommends a clear framework that includes input from medical practitioners, manufacturers, and experts. The Board should use "clinical appropriateness" as the standard for decision-making and exclude cost from consideration. The draft memo should be revised to balance transparency with safeguarding confidential information. Stakeholder input is also crucial in the PDAB's processes, including providing manufacturers with opportunities to review and comment on data.
CANN: CANN is requesting materials from the Federal Trade Commission and the New York Times to be submitted to the Board for review as educational materials. The FTC's first PBM report highlighted how PBMs profit from generic medications and manipulate the Board's weighted considerations. The New York Times has covered the destabilizing impact of the IRA's insulin cap among Federally Qualified Health Centers, raising out-of-pocket costs for patients. CANN raised this issue in its first public comment letter to the Board, but it has remained unaddressed.
Act Now Colorado: Patient and disability advocacy groups for chronic and rare disease patients contest the Colorado Prescription Drug Affordability Board's use of Quality Adjusted Life Years (QALYs) in their decision-making. The groups argue that the Board's actions violate state and federal laws, including CRS Section 10-16-1407(3), which prohibits QALYs, and fail to address patient input as required by CRS Section 10-16-1406(h).
They express concerns that the use of QALYs discriminates against individuals based on disability and age, as QALYs assign a lower value to the lives of those with disabilities or older age. Despite public comments and concerns raised about the discriminatory nature of QALYs, the Board adopted a rule allowing the use of QALYs in affordability evaluations, while prohibiting their use in determining upper payment limits. The Board's decision is unlawful, arbitrary, and capricious, and violates federal and state anti-discrimination laws. Immediate action is needed to rectify the violations and avoid potential legal action if the Board fails to comply.
Comments will be limited to 2 minutes per person or organization.
Oral comments were provided by Jen Laws, CANN, Leah Lindahl, HDA, Amy Goodman, CBSA and Amanda Boone, CF United.
Upcoming meetings
The next PDAB meeting will be March 7, and will begin the Enbrel UPL rulemaking.
Colorado PDAAC meeting, January 23, 2025
Director updates & PDAAC business - Upper Payment Limit – Benchmark Data Memo
Upper Payment Limit – Benchmark Data Memo
The director reviewed the upper payment rulemaking structure using slides from the Colorado PDAB meeting. This includes the data that they will be receiving. The PDAB is seeking edits to the data submission guide from the PDAAC. Enbrel rulemaking was delayed until March.
The director reviewed the Benchmark Data Memo slides. [This is in the slides (pages 3-21)]
Comments from PDAAC members:
Leah Lindahl, from the Healthcare Distribution Alliance, is concerned that wholesaler information is not fully understood by the PDAB members. She was seconded by Fayez Azeez.
Gail deVore asked for a glossary and time spent on developing definitions in documentation.
Katelin Lucariello is concerned about definitions and understanding of rebating, R&D risks and patient assistance.
Director Cummings covered data for upper payment limit consideration:
-
- reasonable pharmacy fees
- removing QALYs information from data provided
Comments from PDAAC members:
Dr. Brett McQueen, CU-SSPPS, asked how the cost benefit analysis will address the calculation of benefit if it is excluding QALY.
Gail deVore asked about “or similar measure” – What does this mean? Can it be defined? It seems too broad.
Dr. Bob Mulch, AARP volunteer, said that for the sake of efficiency they shouldn’t collect dispensing fee data.
After describing the prescription drug and utilization data that will be pulled for the affordability review and benchmarks, Fayez Azeez asked how net price is calculated. Staff answered that it is data point calculated by SSR Health. They are not able to break out rebates, 340b discounts, or other discounts.
Out of pocket spending data, retail discount amounts and wholesale acquisition cost (WAC) were described. Additionally, information for drug shortage, impact to older adults and people with disabilities, and stakeholder input were described.
Dr. Kimberley Jackson commented that the definition of disability is too vague. This was seconded by Gail deVore.
Director updates & PDAAC business - Upper Payment Limit – Draft Data Submission Guide (DSG)
Draft Data Submission Guide (DSG)
The staff reviewed the data submission guide (see materials).
Regarding confidentiality:
Katelin Lucariello, PhRMA, commented that the quality of data will be hindered by rushing the process and asked how improper disclosure will be prevented. Staff asked her to provide methods for providing confidential information in the best manner for her. Also, the members need schedules of when specific data will be discussed so that the correct data experts can be present for those meetings. This was seconded by Marc Reece, CVS Health.
Leah Lindahl asked about the protocol around the use of the data, i.e. a FOIA request? She wanted the PDAB to provide clarity.
Cummings responded that they would build a document that answered to these questions.
Regarding submissions impacting older adults and people with disabilities:
Staff pointed out that effectiveness means clinical, not economic, effectiveness.
Bob Mulch wanted to remove the term effectiveness. Also wants to add the difficulty for older people to obtain rebates.
Regarding QALYs , Katelin Lucariello said that there should be a commitment to reviewing underlying research to insure that QALY analysis isn’t buried in it.
Gail deVore said that patient assistance programs are separate from coupon programs and that that needs to be parsed in the data. This was seconded by Nathan Wilkes, Healthcare for All Colorado.
Brett McQueen, spoke regarding Appendix A, suggesting that staff redact QALYs but include all of the other outcomes because otherwise you would not be looking at the full effectiveness of a drug.
Dr. Richard Miranda asked how do CMS negotiations impact the UPL?
Dr. Pan suggested getting information from the patient side regarding alternative medications for conditions.
Regarding manufacturer submissions:
Katelin Lucariello commented that Individual companies will have different levels of comfort submitting information. Focusing on manufacturing costs ignores years of R&D, post medical surveillance and the time and risk to bring a drug to market.
Fayez Azeez said that it was unlikely that manufacturers would feel comfortable providing that information and that might be better to build the cost analysis themselves by going directly to the API, packaging and other vendors without having the manufacturer actually give it.
Brett McQueen suggested asking the manufacturers why they had changed the price of drugs over time.
Regarding wholesaler submissions:
Leah Lindahl commented that wholesaler manufacturer contracts are complicated, and that the data requested doesn’t cover the complexity of the system.
Katelin Lucariello said that much of the data is highly proprietary.
Fayez Azeez said that he sees the data, as a manufacturer, but getting access to that data would have to come from the manufacturer.
Regarding carrier and PBM submissions:
Marc Reece commented that a PBM is negotiating on behalf of a carrier’s members and will determine a formulary placement and then cost sharing.
Dr. Pan refered to alternative funding, i.e. smaller carriers are focusing on getting medication from foreign, non-US approved sources.
Katelin Lucariello pointed out that UPLs have other impacts besides lowering premiums that haven’t been considered.
Regarding pharmacy/provider submissions:
No comments were provided.
The staff asked for written comments on the data submission guide.
PDAAC 2025 meeting schedule
Next PDAAC meeting is to be determined. Next PDAB meeting is March 7, 2025 at 10am MT.
Public comment
Dr. Richard Miranda, PDAAC member, asked whether UPLs expire, whether they apply to drugs after they become generic, and whether there is a mechanism for appeal. The staff said they will follow up.
Amanda Boone, Co-Founder of CF United: Alternatives to QALY based analysis need to be considered.
Bridget Serritt, Founder of Advocates for Compassionate Therapy Now: Also says that QALYs are discriminatory. Definition of disability is problematic and needs clarification.
Maryland PDAB meeting, January 27, 2025
Opportunity for public comment
After routine business, the public was invited to comment. Written comments were posted to the website.
- Two letters from Tiffany Westrich-Robertson, Ensuring Access through Collaborative Health (EACH) Coalition and Patient Inclusion Council (PIC)
- Flyers from Public Citizen regarding Jardiance and Farxiga
Three people spoke:
- Jane Abernethy commented on the cost of Jardiance and Farxiga and supported lowering of cost of medicines for diabetes, kidney and heart disease.
- Len Lucchi, a Jardiance patient, spoke about his medication being too expensive.
- Peter Maybarduk, Access to Medicines Director, Public Citizen opined that medication is too expensive and drug companies spend too much money on themselves and advertising.
Final action—Amendments to COMAR 14.01.04.05 Cost Review Study
Staff Presentation by Andrew York
Regulations Process
- The Board approved proposed amended regulations at the September 10, 2024 board meeting.
- Proposed regulations were published in the November 1, 2024 Maryland Register.
- Comments were accepted through December 2, 2024.
- Three comment letters were received.
- These regulations were also approved as Emergency Regulations with an effective date of November 14, 2024.
Current Amendments
(deleted language is crossed out, new language is underlined)
Amends came in three main categories: (1) clarify analyses and data sources, (2) Add Cost Review Study Factors, (3) NEW: Track UPL Action Plan –Preliminary Determination that Drug Will Lead to Affordability Challenge and Identify Circumstances.
Policy Section .05B(2) Analyses and Data Compilation
(2) These data and analyses may be:
(f) Derived from the MCDB, any claims set of the MCDB, and any other databases containing relevant information; [or]
(g) Derived from reports generated by U.S. governmental entities, state governmental entities, foreign governmental and quasi-governmental agencies, and U.S. and foreign non-profit organizations; or
(h) Derived from quantitative and qualitative data collected by Board staff.
Summary of comments:
Commenters objected to “state governmental entities” and “any other databases” and the board considered and rejected the objection.
Policy Section .05C(1) Factors Considered in Cost Review Study
Added factors considered in the cost review study:
(c) Therapeutic Alternatives: (iii) The utilization, costs, and out-of-pocket costs for therapeutic alternatives;
(d) Patient Access: (iii) The current or expected dollar value of drug-specific patient access programs that are supported by the manufacturer for the drug product under review and the policies surrounding and implementing such programs;
(g) Additional Board Factors:
(iii) [In the case of generic prescription drug products, the] The number of pharmaceutical manufacturers that produce [the] prescription drug [product] products that are therapeutically equivalent to the drug product under study;
(xi) Analysis of the market context of the prescription drug product including the prescription drug product’s lifecycle management, patent management, regulatory exclusivities, and product [copying] hopping;
(xii) The utilization and pricing of therapeutically equivalent drug products;
(xiii) Analysis of the impact of state and federal regulatory and compliance issues related to the prescription drug product;
(xiv) Input from state and local governmental entities and the entities’ contractors such as health plans and plan administrators;
(xv) Impact of the utilization and spending for the prescription drug product on public budgets and comparison of the spending on the prescription drug product to relevant benchmarks;
(xvi) Analyses and research including literature review by Board staff in response to information submitted by an entity under Regulation .04 of this chapter, or through any public comment or public input procedure;
Summary of comments:
Overall: Commenters noted the degree of detail used to describe the Board's affordability challenge factors and how they may be weighed (e.g., patient access programs, impact on public budgets, state/federal regulatory/compliance issues, market context, and literature review).
Therapeutic alternatives: Commenters raised questions about how therapeutic alternatives are considered, and related data. One commenter suggested that clinical appropriateness should be considered before drug costs.
Market context: One commenter requested removal of “product hopping.”
The Board's role in ensuring compliance with all federal and state laws relating to prescription drugs is being expanded, according to a commenter.
The Board should consider patient out-of-pocket costs, patient experience, manufacturer discount programs, cost offsets, and the impact of benefit design on these costs.
Policy 0.5D Board Action
At an open meeting, the Board may: (5) Preliminarily [Determine] determine whether:
0.5F Preliminary Determination
(1) In accordance with §C of this regulation, the Board may make a preliminary determination of whether use of the prescription drug product has led or will lead to affordability challenges for the State health care system or high out-of-pocket costs for patients.
(2) A preliminary determination is non-final and subject to revision and modification.
(3) Preliminary Determination of Affordability Challenge.
(a) Board staff shall prepare a draft of the preliminary determination cost review report that summarizes the information considered by the Board in conducting the cost review study, the Board’s deliberations, the circumstances or indicia reflecting the affordability challenge, and the Board’s preliminary determination.
(b) The public may comment on the draft of the preliminary determination cost review report.
Cross-Reference for Policy .05A Cost Review Study - Board Action.
(3) Identify the circumstances under which the prescription drug product has or will lead to an affordability challenge to the State health care system or high out-of-pocket costs to patients under §A(1) of this regulation.
Policy.05G Final Determination Concerning Affordability Challenge and Final Cost Review Study Report
[F.] G. Final Determination Concerning Affordability Challenge and Final Cost Review Study Report.
(1) The Board may vote to finalize the preliminary determination and approve the draft cost review report as final.
(2) The Board’s determination of whether a prescription drug has or will lead to an affordability challenge is not final until the final cost review report is adopted by the Board.
Summary of comments on proposed amendments - Preliminary and Final Determination (COMAR 14.01.04.05F and G)
State and federal drug regulation and compliance analysis: This was seen as expanding the Board's role to include compliance with all federal and state prescription drug laws.
One commenter requested a 60-day comment period on the draft cost review study report and more detail on the preliminary determination.
Recommendations
The Board voted to adopt Amendments to COMAR 14.01.04.05- Cost Review Study as FINAL.
Cost Review Study
- Staff Presentation—Overview of Cost Review Study process and opportunities for public input
Document here. There will be a stakeholder council meeting at end of February for public feedback. The Next PDAB meeting is March 24, 2025. The Board is considering ad hoc meetings to move forward. - Closed Session to consult with counsel and receive legal advice regarding Cost Review Study, under GP § 3-305(b)(7)
Administrative update
A series of regulations in the Maryland regulation are currently in the policy review process. Comments are due February 10, 2025. The Board may want to schedule ad hoc meetings before the next scheduled meeting. Board staff will be advising on MD House Bill 424 cross-filed with MD Senate Bill SB0357, the Prescription Drug Affordability Board - Authority for Upper Payment Limits (Lowering Prescription Drug Costs for All Marylanders Now Act). There will be a hearing in the House at 1:00pm on February 6.
Bill synopsis: Requiring the Prescription Drug Affordability Board, under certain circumstances, to establish a process for setting upper payment limits for all purchases and payor reimbursements of prescription drug products in the State that the Board determines have led or will lead to affordability challenges; authorizing the Board to reconsider an upper payment limit for a drug that becomes a current shortage; altering requirements related to the setting of upper payment limits by the Board; etc.
Oregon PDAB meeting, January 15, 2025
After minutes were approved, the executive director presented his report.
Executive Director’s program update
After routine items, Executive Director Ralph Magrish said that staff were looking into commercially available products or services to provide access to varying data points that would address frustrations expressed by board members and through the public comment process.
Beginning in February, there will be a standing agenda item about legislative updates specific to bills related to the pharmacy supply chain, coverage and reimbursement of prescription drugs. This update will be provided directly from DCBS policy staff.
Oral comment
The board received six written comments from Dharia McGrew from PhRMA, Rainier Simons and Jen Laws from CANN, Tiffany Westrich-Robinson from EACH Coalition, Tony Coelho from Partnership to Improve Patient Care and Dr. Harry Gewanter with Let My Doctors Decide Action Network.
Oral comments were provided by:
Eric Lohnes, PhRMA:
PhRMA requested the board to reconsider its public comment timeline. The current practice of posting materials one week in advance and requiring public comment on Sunday mornings only allows stakeholders two to three days to read, evaluate, and respond to the complex issues, which is insufficient time. They suggested amending the board's policies to commit to posting meeting materials at least two weeks prior to each meeting.
The technical feedback on the carrier data call template suggested that it didn’t make sense in the reality of the supply. The call asked plans to report distinct values of price concessions from manufacturers and pharmacy benefits managers (PBMs). Manufacturers typically pay rebates directly to PBMs, which then pass them on to health plan clients or employers. This may have contributed to confusion in affordability reviews last year. The board members had no information on rebates, but some affordability reviews had information titled PBM Concessions. We ask that you look at both the carrier data call and the review template to clarify these terms.
Ben Hughes, Health HIV:
Funds from clinics and CBOs support the broader community through contracts with health departments and service providers. Implementing upper payment limits without fully accounting for the interconnected system risks undermining access to medications and services for people living with HIV. To address these concerns, a rigorous data framework is proposed to ensure decisions on UPLs are supported by accurate, Oregon-specific data.
Hughes asked that the board pause and evaluate UPL implementation until federal guidance stabilizes, prioritizing reforms with proven efficacy to ensure Oregonians can access necessary medications. Implementation of UPLs should be informed by comprehensive data for stakeholder engagement.
Rainer Simons, CANN:
Mr. Judge's conflict of interest led to his recusal from voting on policy recommendation number 5, but he was active in the discourse leading up to the votes. He should have also recused himself from voting on policy recommendation 9 regarding dispensing fees and reimbursements, as it was highly related to his employer's profits. However, he provided significant input to the discourse concerning the measure and ultimately voted against it. While the law may not forbid commentary towards votes on issues a board member recuses themselves from, doing so is an ethical violation and is not proper management of trust in terms of the public interest.
Pharmacy protections should be prioritized over affordability, as functionally inaccessible medication may not be affordable if the pharmacy is closed or providers are unavailable.
Board annual policy review
The board approved policies to guide its work when it was established in June 2022.
The policies are posted on the PDAB website. Every year, the board reviews the policies for possible amendments. See Title: Policies and Procedures.
Policy Number 01, “Policies and Procedures” have the following items up for board vote:
- Executive session - The removal of the last sentence under executive session that states that electronic records for the executive session are securely stored. There's no recording of the executive session so this can be deleted.
- Meeting attendance, absences, and participation - Add additional clarifying words to indicate that on average board members are expected to participate approximately 10 to 15 hours monthly, including board meetings, meetings with staff, and board material reviews.
- Board Issued iPad - Board members need to login into state issued iPads at least every 45 days and change passwords every 90 days. Board members will receive email or text messages for those reminders.
- Coordinating with other entities: Board members are not obligated to speak about board business outside of board meetings and may delegate those requests to the staff if needed.
Also, board members must disclose at the beginning of each board meeting any meetings or work conducted with entities or individuals related to board activities since the last board meeting. This can include serving on other boards or committees, or engaging in business matters related to pharmacy supply chain.
Board members felt it would be “too much” to discuss all of their daily work at the beginning of every meeting, and that it would be too difficult to apply. Board chose to send the commentary back to the DOJ to review it and come up with language before the policy is adopted.
Language for the board and DOJ to review: “[Whenever possible] If a board member intends to abstain from voting on any matter or section under consideration, they must declare their intent to abstain prior to discussion related to the vote. A declaration of abstention [may] include a brief explanation such as a potential conflict of interest or other relevant reason, to ensure transparency and maintain trust in the decision-making process.”
Policy Number 04, “Public Comment” had the following item up for board vote:
2) Policy Statement: Due to board meetings and time constraints only one person per organization will be added to the list of public comment speakers for each board meeting.
The board discussed the deadlines for written and public comments before meetings and public concerns about having insufficient time for review. The existing policy was that people had to submit written comment 72 hours before a PDAB meeting and sign up for oral comment no later than 24 hours before. They changed the written comment deadline to 48 hours before meetings.
The board voted to approval all policy changes except for Policy Number 01, “Policies and Procedures” 16, which was referred for staff to discuss with DOJ and the Ethics Commission.
Board review and vote on carrier data call template
The board will begin the affordability review process in upcoming months to prepare the Department of Consumer and Business Services. The board reviewed the draft template included in today's agenda materials and in PDF on the website. The board will select a subset list of drugs and insulin products from the preliminary list, which are then sent to health insurance carriers for additional information.
See template in the agenda. The template is an excel spreadsheet that gives instructions for health insurance carriers to fill out. Tabs include company information, data limitations and notes, claims and cost, plan design and price concessions.
Review of datasets and OER 925-200-0010 criteria for upcoming affordability reviews
One purpose of the PDAB is to conduct affordability reviews on prescription drugs and insulin products. In this meeting, the board reviewed the preliminary list of prescription drugs and insulin for affordability review, which will begin in the coming months. The lists are posted on the PDA website, and links are included in today's material packets.
Previously the board completed Phase 1:
The board began Phase 2, selecting prescription drugs for affordability review. See slides below.
Staff shared the spreadsheet with detailed drug and carrier information.
Carrier Prelim RX List 24-25 (screenshot, not whole list)
The board cannot review any drugs associated with rare conditions or that have orphan designation. Once those medicines are filtered out, there are 95 drugs left to review.
- Board member question: The biosimilar Humira was initially listed without an orphan designation, but it was later discovered to have one for at least one indication. The board staff should consult with a clinical consultant to verify the accuracy of the data.
- Board member question: Are physician samples included in the data? No.
- Board member question: How did we use guidance last year when making this decision around therapeutic alternatives? And the number to consider that there's market competition with a number of therapeutic alternatives? I mean, so if we're trying to pick 5 or 7 or 3? What did we as a board land on for that number?
Answer: There was never any kind of goal or target of how many therapeutic alternatives would be identified. We retain a clinical consultant, a doctor at a pharmacy who's on faculty at Oregon State University College of Pharmacy to assist us in identifying those.
Drugs part of IRA CMS negotiation list:
One of the decisions from the first review was to remove any of the drugs that were being reviewed under the Centers for Medicare & Medicaid Services (CMS) negotiation list. The CMS will be releasing a new list in March of the new set of drugs to review. There's no obligation for the board to remove those drugs knowing that the financial impact really won't be seen until 2027 or 2028.
Insulin list:
Screen shot of spreadsheet (posted online)
The board discussed how to pick drugs, and considered filtering the list by the following:
- Remove all drugs with orphan drug designation
- Drugs on the IRA
- Generics
- Drugs that have generics
- Filtered by most costly
- Sort by number of carriers.
The board discussed inaccuracies in column information: conflation of “therapeutic equivalent” vs. generic, biosimilar, and therapeutic alternatives, and asked for 340b and FQAC data.
The next PDAB meeting will be February 19, 2025.
Washington PDAB meeting January 15, 2025
PDAB Director's Report, presented by Mike Neuenschwander
MaryAnne Lindeblad is resigning from the board due to a new position as the acting director for the Healthcare Authority.
The Board is creating industry data submission forms.
At the December 10th Advisory Group meeting the Board broached discussion about the process for selecting the drugs.
The lawyer (Michael, last name unavailable) explained the legal term “arbitrary and capricious” by recounting the legend of King Solomon splitting the baby in half, and by explaining that 65 as the age for the receipt of social security benefits was based upon life expectancy of less than 65 [which is false – this is based upon average life expectancy including child mortality. According to the Social Security Administration, those that lived to adulthood in 1940 lived until 73 and 75, on average].
According to the lawyer, an agency action is found arbitrary when certain factors need to be considered and haven’t been. Mike Neuenschwander summarized the relevance to the PDAB: “The general gist is that making sure we have methodologies that we've developed in terms of creating the drug list, in terms of how we're prioritizing the drugs, not going against the legislation or our rules, and that we have a reasoned decision of how we're coming to these selections.”
Neuenschwander reiterated that as long as “we stick to our methodologies, that we’re following our processes, that we’re creating reasoned decisions...I think we should be good.”
Vote on Drug Selection Policy
Motion: Drug selection policy accepted unanimously. Method for selection prescription drugs for affordability review here.
Review Data Dashboard
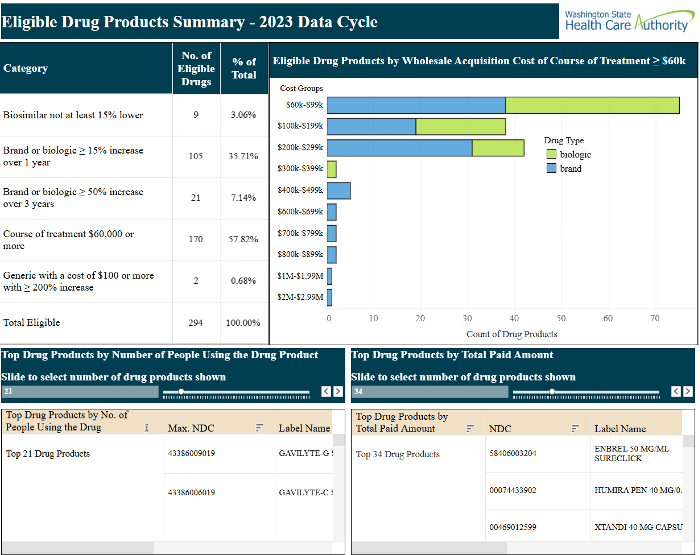
Staff provided an overview of the data dashboard, which is publicly available for review.
This is a screenshot of the Eligible Drug Products Summary – 2023 Data Cycle page.
The board discussed review procedures. For example, would they do one drug in all tablet sizes? Does the database allow aggregation of all doses of one drug?
The board pointed out the database doesn’t include all the data for each drug for all doses, that you can’t filter by diagnosis and that summing and averaging functions in the database didn’t work. For the prioritized list, they would like to see the top 25 with specialty and non-specialty drugs combined. The board is trying to address public comments on the dashboard and the “downstream” impacts of their data choices.
Discuss Number of Drugs on Which to Conduct Affordability Reviews, Create Shortlist for Eventual Vote
(This was an open floor for Advisory Group members to discuss their rationale)
Top Advisory Group Recommendations
| Drug | Number of Advisory Group Votes |
ICER Analysis |
| Enbrel | 4 | Y |
| Cabometyx | 3 | Y |
| Xtandi | 3 | Y |
| Taltz | 3 | Y |
| Xeljanz | 2 | Y |
| Orencia | 2 | Y |
| Humira | 2 | Y |
The Advisory Board provided commentary on their thought process, including staff ranking and number of patients impacted by drugs. Quantifiable metrics should be used. Should consider how other states are doing analysis.
Board members are concerned about the budget, which is uncertain until April. Resources may be constrained. Board chair suggested a “more modest approach” from a staffing point of view. The goal was to decide short list of drugs to review in March, with the expectation of reviewing one or two drugs at a time.
Board members were concerned with the ability to aggregate branded and generic versions together, including by ingredient level. Discussion centered on aggregation and sorting in order to address board concerns.
Public Comment
Public comments:
-
Sue Birch, Director of the Healthcare Authority thanked the board for their work.
- Daria McGrew, Director of State Policy, PhRMA asked that members be of cognizant of list prices vs WAC and payer payments and whether they’re in the dashboard. Will take the dashboard back to experts for review with technical comments.
Next Meeting
The next meeting of the Washington PDAB will be March 19, 2025.
Standout resources and coverage
"Specialty generic drugs: A growing profit center for vertically integrated pharmacy benefit managers," Federal Trade Commission. January 2025: This report expands on FTC staff’s initial findings regarding specialty drugs published in a July 2024 staff report titled “Pharmacy benefit managers: the powerful middlemen inflating drug costs and squeezing main street pharmacies” by examining the increasing importance of specialty drugs to the three largest PBMs and their affiliated pharmacies. The report finds that the Big 3 PBMs impose significant markups on a wide array of specialty generic drugs.
"Insulin prices dropped. but some poor patients are paying more," New York Times, January 16, 2025: A law that coaxed companies to lower the price of drugs came with a little-known consequence: smaller discounts for low-income health clinics.