Compounded weight loss injections — knock-offs are not the same
Who says legally-compounded versions are not as safe as the FDA-approved products? The Food and Drug Administration.
In November 2024, the Food and Drug Administration (FDA) issued "Compounding and the FDA: Questions and Answers," warning that
Compounded drugs should only be used in patients whose medical needs cannot be met by an FDA-approved drug. Unnecessary use of compounded drugs may expose patients to potentially serious health risks. For example, poor compounding practices can result in serious drug quality problems, such as contamination or a drug that contains too much or too little active ingredient. This can lead to serious patient injury and death.
FDA hasn't reviewed the products compounding pharmacies are making for effectiveness, safety or manufacturing quality prior to being sold, as they do with approved drugs. When the FDA approved Wegovy for weight loss, it was approving Novo Nordisk's specific product after evaluating results from studies the company ran using specific ingredients, doses and delivery methods. Those tests don't prove that compounded medicines, which have different formulations, will work the same way.
PSM is running an ad to warn consumers about the risks.
When PSM talks about knock-off versions of FDA-approved medicines, we're talking about alternatives that might be counterfeit, illegally compounded, or legally compounded but with a lower safety profile.
- Learn more about the FDA's work fighting counterfeit and illegally compounded weight loss injectables.
- Read the letters we sent to Fox Broadcasting and the Food and Drug Administration explaining our concerns with the Hims & Hers Super Bowl ad, which promoted compounded weight loss injectables without mentioning their potential risks or side effects.
Why do we have compounded medications then?
Compounded medications fill two important needs in our medicine supply (and we don't want to live without them):
- They allow people with unique needs that aren't met by the commercial market to have a medicine formulated just for them. One example of this would be a medicine that only comes in pill form for a patient that cannot swallow pills.
- They allow the production of medicines that the FDA has declared are in shortage to ensure medical needs are met until the shortage can be resolved.
The FDA is clear that because of the lower safety profile of these medicines, they should not be used except as a last resort. That's not the same as being generic. Generic medicines are FDA-approved. FDA must find that the active ingredient in a generic is the same as the brand name drug, based on scientific evidence, and they are subject to the same manufacturing standards as branded FDA-approved medicines.
Most Americans have no idea what it means for a medicine to be compounded, and don't understand they are accepting a lower level of safety.
Additionally, researchers have found that shady vendors sometimes sell compounded medications and misidentify them as generic.
Online advertising of compounded glucagon-like peptide-1 receptor agonists was published in JAMA Health Forum in January 2025.
Confusion abounds for consumers
It is often difficult for patients to find out where their compounded weight loss injectable medicines are coming from. Consumers looking for compounded medicines have to navigate through a crowd of both illegally and legally compounded medicines.
Once they do find a legal, licensed compounding facility, there are still unresolved questions about where compounders are sourcing their active ingredients, whether anyone is safety-testing those products, and whether they are manufacturing them in a sterile environment.
Just recently, the FDA sent a warning letter to a facility that makes compounded medication and the first of many citations was for obtaining ingredients from unauthorized suppliers. ("Your facility compounded drug products using a bulk drug substance from [redacted by FDA], which is not a registered establishment under section 510 of the FDCA.")
In the U.S., regulated semaglutide and tirzepatide are solely made by Novo Nordisk and Eli Lilly for the production of Ozempic, Rybelsus, Wegovy, Mounjaro and Zepbound. In 2024, both Novo Nordisk and Eli Lilly said that they were not supplying compounders with the semaglutide and tirzepatide they are selling to their customers.
Both companies have found contaminated or counterfeit compounded diabetes and weight loss injectables. In May 2024 Nordisk filed suit against a Tennessee-based company that it says sold counterfeits: their "semaglutide" injectables did not contain any semaglutide. Eli Lilly has filed similar lawsuits.
FDA has previously expressed concerns about the semaglutide sodium and semaglutide acetate some compounders are using in their knock-off injectables. These products are not the same as semaglutide and tirzepatide and haven't been studied for safety or effectiveness.
What do you mean when you say compounded medications aren't clinically tested or have the safety and quality assurances of the real thing?
FDA-approved medicine goes through an extensive process. Essentially, the entire manufacturing process is inspected from start to finish and the medicine the process creates is tested for safety and effectiveness. This is what it takes to get a medicine FDA-approved, and it's rigorous, which is why it's the gold standard of the world. This FDA video explains the process pretty well.
Very little of that process is applied to compounded medications, which is why the FDA recommends them only as a last resort and warns Americans they are not as safe as branded or generic FDA-approved medicines.
Additionally, there are other elements which we are highly concerned about that include:
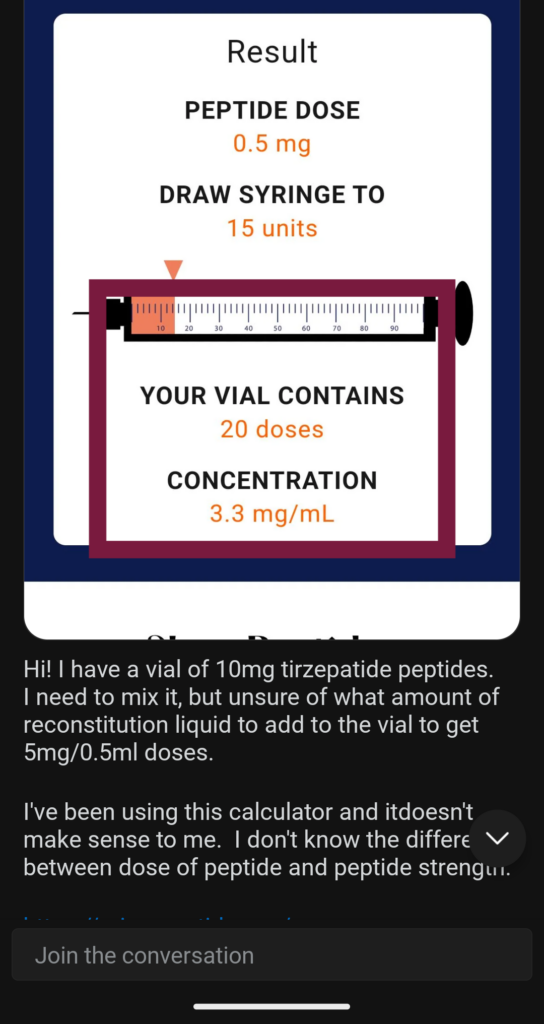
- Unclear labeling: The labels for compounded medications don't have the same requirements as those for FDA-approved medications. You can see the problems if you look at any forum of people using compounded weight loss medicines. They have a great deal of trouble deciphering how much of the medicine to inject. You can see some examples of that below in our section, "Why is the FDA worried about dosing errors?" The FDA has alerted health care providers, compounders, and the public about dosing errors with compounded versions.
- Lack of traceability: Compounded medications are not required to have the serial numbers (per the Drug Supply Chain Security Act) that FDA-approved medicines have. This serial number system is crucial to protect patients from criminals putting suspect medication into the drug supply chain.
When your local pharmacist was compounding the medicine for you in the store and handing it to you, that was no big deal. But now facilities you will never see are compounding medicines and delivering them to you after a prescription by an provider you may never see in person, either. Traceability is an issue, and nothing short of legally-mandated traceability will suffice.
It is hard to make safe injected medicine?
Manufacturing sterile medicine is a demanding, complex process that is particularly important when you are injecting a product directly into your body. Producing sterile medicine has been a problem for many compounding pharmacies, and when there have been sterility issues, it has caused harm to patients.
In 2024, compounding facilities in Kansas, Pennsylvania, New Jersey and California received FDA warning letters after inspectors identified problems with sterility in their facilities. All of those facilities made sterile injectables, one of those facilities made semaglutide and tirzepatide, and one had acquired at least one bulk drug from an unregistered supplier. FDA also specifically warned patients not to use compounded drugs from a California compounding pharmacy.
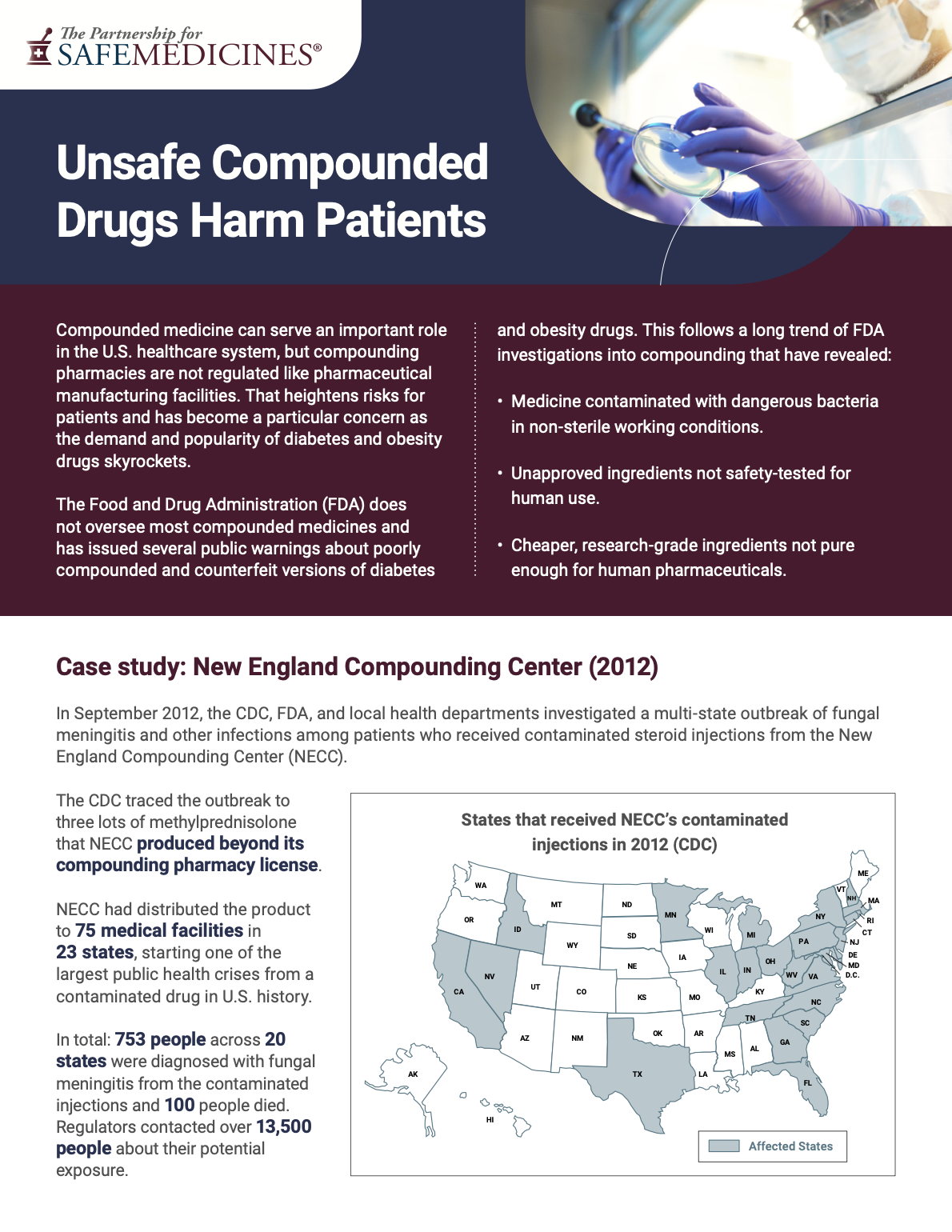
In 2012, contaminated steroid injections from New England Compounding Center (NECC) triggered an outbreak of fungal meningitis that sickened more than 750 people and killed more than 100 of them. Since then, problems with faulty compounded medicine have caused infections, blindness, and even death.
Has anyone really been hurt by these compounded injectables?
Yes. As of November 30, 2024 the FDA had received almost 400 reports of adverse events from compounded semaglutide and over 200 from compounded tirzepatide, some of which may be related to dosing errors. Because federal law doesn't require state-licensed pharmacies to submit adverse events to FDA, it is likely that adverse events from compounded versions of these drugs are underreported. More incident history can be found in our handout "Unsafe Compounded Drugs Harm Patients."
Why is the FDA worried about dosing errors?
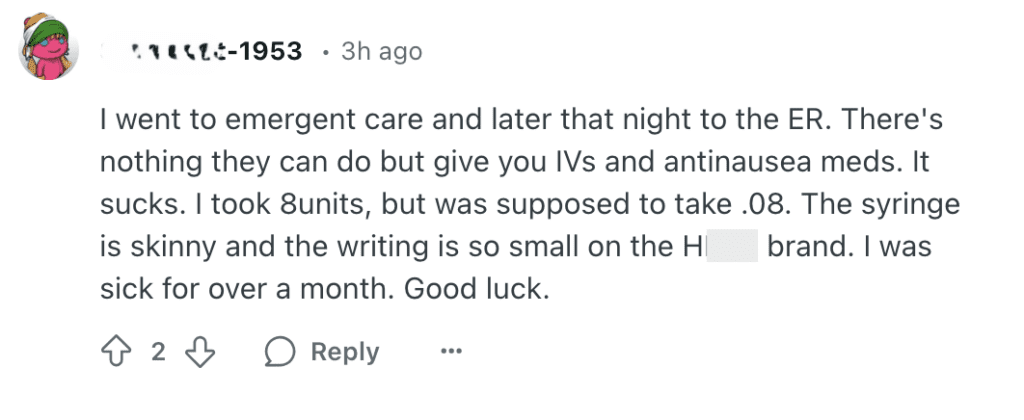
The FDA says it has has received multiple reports of dosing errors with compounded injectable semaglutide products—both on the part of patients and health care professionals. Some of these required hospitalization.
Monitoring online conversations about the drugs, it's easy to see why. The FDA-approved versions of these drugs come with prefilled injectors but compounded versions often come with a vial and a syringe. Some illegally compounded products even come as a powder that needs to be mixed with a liquid and THEN injected. Reddit forums are filled with people asking for help determining doses for compounded injectables.
These are just a few of the Reddit posts we found in January and February 2025. (Click to enlarge them.)