March 17, 2025: A Texas Pharmacist gets 17 years for $145m skin cream billing fraud
Major Stories
A Texas pharmacist billed government programs millions for creams compounded by untrained teenagers.
A federal judge sentenced former pharmacist Dehshid “David” Nourian, of Plano, Texas to over 17 years in federal prison and to pay a forfeiture of $115 million for health care fraud. With co-defendants, Nourian, a co-owner and -operator of three pharmacies in the Dallas area, paid doctors millions of dollars to prescribe compounded creams to treat scars, wounds, and pain to injured federal workers. Between May 2014 and March 2017 the pharmacies billed as much as $16,000 for prescriptions that cost $15 to make, and the Department of Labor’s Office of Workers' Compensation Programs and Blue Cross Blue Shield reimbursed more than $90 million for them. According to the Department of Justice, patients testified at trial that the compounded creams, which were made by untrained teenagers, were ineffective and caused painful rashes.
Patient safety issues in the GLP-1 space this week
The Federal Bureau of Investigations warned that a Georgia compounder was making weight loss medicines in violations of FDA regulations. The injections contained animal-grade semaglutide and Vitamin B12.
Washington’s Pharmacy Quality Assurance Commission issued a limited stop service to Aequita Pharmacy, which compounds semaglutide and tirzepatide. The pharmacy allegedly used unqualified staff to make products and violated sterile compounding procedures.
The U.S. Food and Drug Administration published a February 2025 letter warning a German company to stop selling Americans unapproved, “research” versions of semaglutide and tirzepatide that are clearly intended for human use.
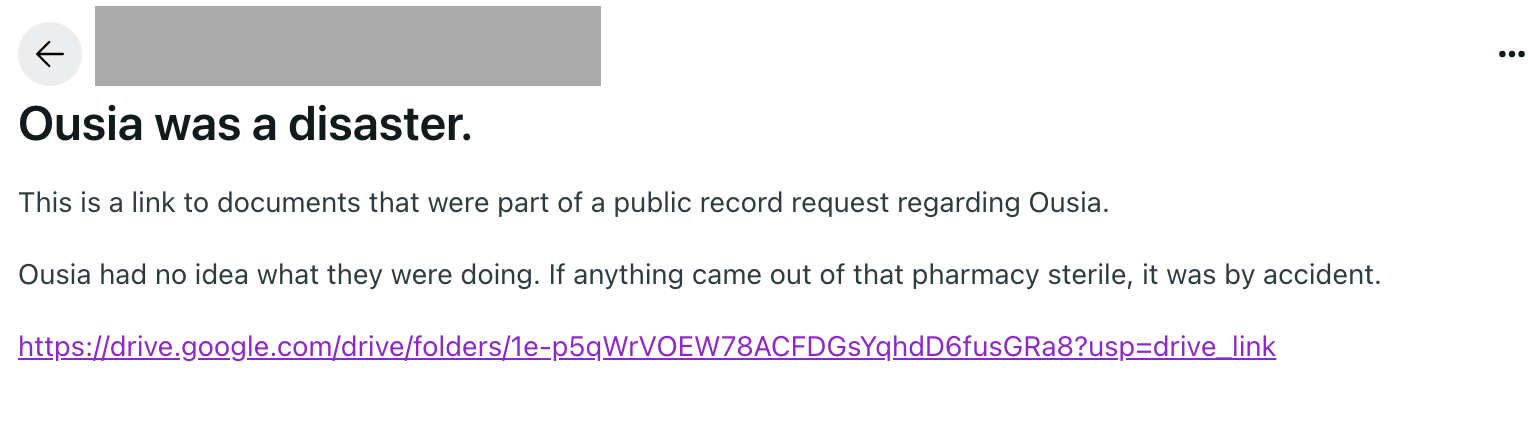
Florida-based Ousia Pharmacy, which provided compounded GLP-1s for telehealth companies, relinquished its license at the end of January after the Florida Department of Public Health filed a complaint about its lack of sterile compounding license.
The pharmacy’s closure continues to trouble patients who had stocked up on their products. Last week, a Reddit user posted inspection reports that detailed non-sterile conditions at the facility, declaring, “If anything came out of that pharmacy sterile, it was by accident.”
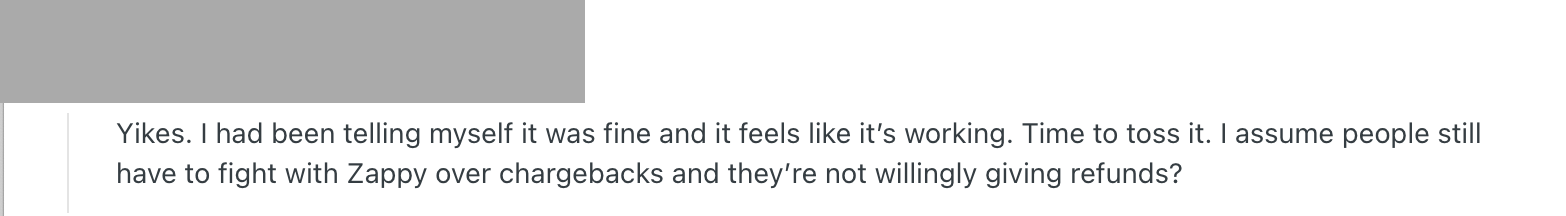
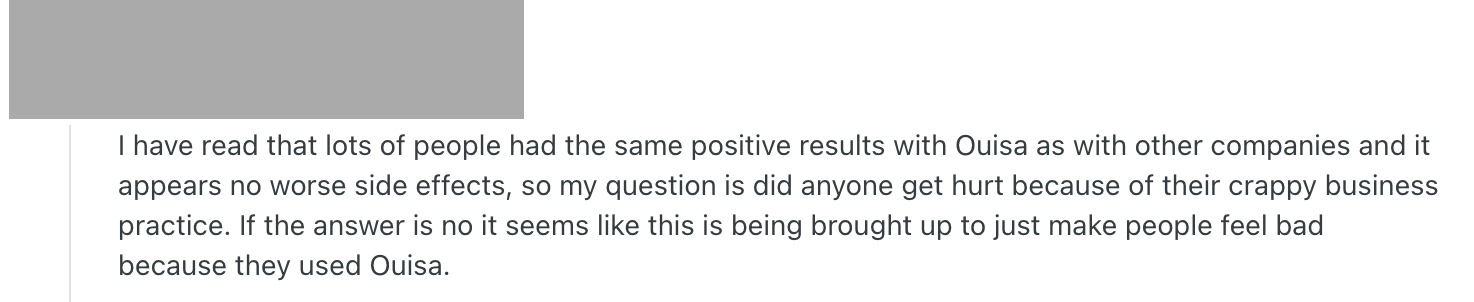
Some people who had been injecting Ouisa products even after the pharmacy’s closure were shocked into tossing them. One suggested that the pharmacy’s products worked as well as others, so the subject was only being raised to “make people feel bad.” Another said they had decided to take their chances on the gray market (importing medicine from foreign vendors themselves) when they saw what Ousia had been selling.
Ousia’s inspection reports (inspector narrative | report) show how complex and exacting it is to compound sterile injections. History shows that non-sterile products can sicken and even kill patients. If you are a prescriber or a patient, guard your safety:
- Only use compounded products when mass-produced FDA-approved medicine won’t meet your needs.
- Make sure the facility making the medicine has an appropriate compounding license in the state where it resides and the state where the medicine will be dispensed.
- Monitor the FDA’s recall announcements and warning letters to keep up to date about potential manufacturing issues.
- And finally, don’t buy medicine from gray market sources. It is difficult to know whether products from unlicensed suppliers are ineffective or harmful, and vendors that are overseas are beyond the reach of U.S. authorities.
Domestic News
Updates to prosecutions related to counterfeit medicines in California, Massachusetts, Oklahoma, and both Carolinas.
The FDA published a December 2024 warning letter to a compounding facility in Texas that sold adulterated products without adequate labeling.
Agents with the Drug Enforcement Administration arrested four Framingham, Massachusetts residents who allegedly imported and sold Brazilian pharmaceuticals and other misbranded drugs, including codeine, tramadol, clonazepam, and morphine.
Ibrahim and Ahmed Shedid of Summerville, South Carolina were each sentenced to 26 months in federal prison and to pay more than $25 million in restitution after they were caught with more than 10,000 bottles of counterfeit Viagra, which they intended to distribute to local convenience stores.
Aaron Michael Thomas of Tulsa, Oklahoma received a 78 month federal prison sentence for introducing misbranded drugs into interstate commerce, maintaining a drug-involved premises, and possessing child pornography. Thomas and co-defendant Darren Doil Means illegally imported pregabalin, xylazine and other drugs from China and sold them on the dark web. A search of their packaging and distribution center in October 2023 yielded more than 270 pounds of 21 different drugs or active pharmaceutical ingredients and an encapsulation machine.
A Texas federal court sentenced John Khuu, of San Francisco, California to more than seven years in prison after he pleaded guilty to money laundering and operating an unlicensed money transmitting business. Khuu and co-conspirators used Bitcoin to launder profits from selling illegally imported counterfeit pharmaceuticals and MDMA pills on dark web markets.
Rockingham, North Carolina resident Adam Meland pleaded guilty to introducing misbranded drugs into interstate commerce for his part in running an online e-commerce website that illegally imported, repackaged and sold drugs commonly prescribed for weight loss, diabetes, erectile dysfunction, and narcolepsy to U.S. customers. A warranted search in Rockingham led to the seizure of hundreds of bags of capsules, dozens of bottles of liquids labeled as steroids and testosterone, firearms, and more than $150,000 in cash.
PIll presses were seized in unrelated fentanyl busts in New York City and Greenwood, South Carolina.
International News
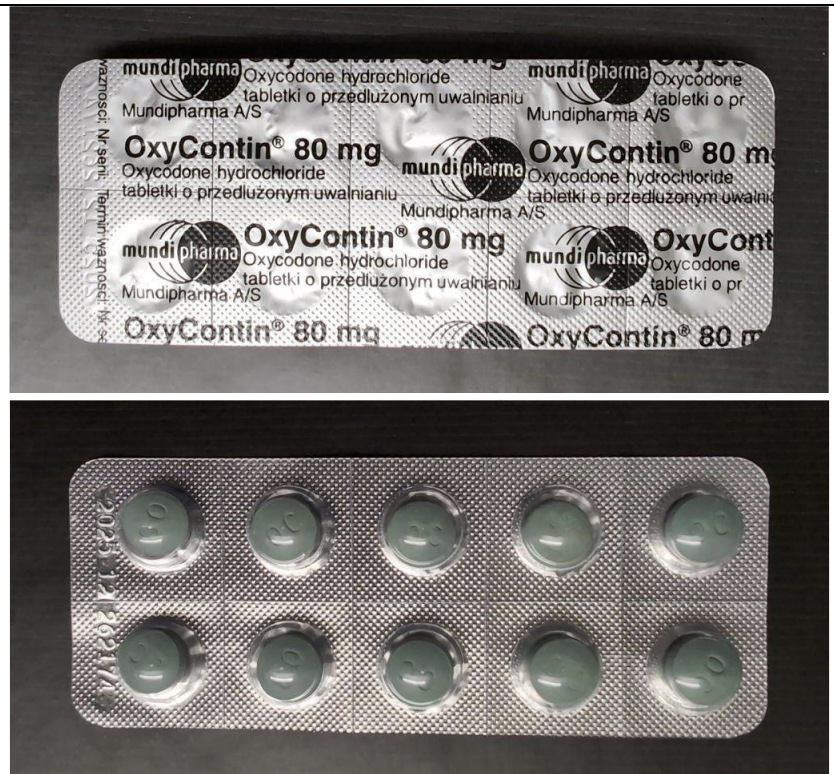
WHO warned about counterfeit Oxycontin made with nitazines.
The World Health Organization issued an alert about counterfeit 80mg Oxycontin found in Switzerland. The pills, which mimic genuine Oxycontin authorized in Poland, contain a synthetic opioid likely to be a nitazene compound.
The Abu Dhabi Department of Health reported adding 18 counterfeit products—chiefly male enhancement supplements and skin creams— to its list of counterfeit medicines, dietary supplements, and cosmetic products.

![Ousia-buyer-3 Reddit comment "The second this pharmacy popped up and I saw how the vials looked and all the craziness around them I knew it was time to go 🩶 [gray heart] and be prepared to handle things myself if they could.](https://www.safemedicines.org/wp-content/uploads/2025/03/Ousia-buyer-3.png)