The U.S. Attorney's Office in the Western District of Washington has charged two Indian nationals with selling Americans counterfeit Keytruda.
Major Stories
The U.S. Attorney's Office in the Western District of Washington has charged two Indian nationals with selling Americans counterfeit Keytruda.
Two Indian men extradited from Singapore at the end of February have been charged with selling counterfeit oncology medicine to U.S. buyers. A 2022 indictment alleges that the men smuggled at least 33 boxes of unapproved Keytruda, as well as other misbranded oncology medicines, into the U.S. between 2019 and 2021. It also lists nine vials of Keytruda that were tested and found to be counterfeit; some had no active ingredient.

Patient safety issues in the GLP-1 space this week
In a February 25 article, Securing Industry wrote about PSM’s recent analysis of FDA import records, which showed that shipments of semaglutide and tirzepatide from unregistered sources were being approved for entry into the U.S.
Hims & Hers announced that it may no longer be able to sell compounded versions of weight-loss drugs because the U.S. Food and Drug Administration (FDA) had declared them no longer in shortage.
Although Hims claimed to be exempt from FDA advertising regulations when they aired last month's Super Bowl ad promoting compounded weight-loss injectables, the company acknowledged the risk of litigation as a result of noncompliance with those regulations in this year’s annual report to the Security and Exchange Commission. Read what PSM’s FDA regulatory and legal counsel had to say about the issue.
A user asked his subreddit whether anyone knew what these particles were.
The best response?
"Given the size and unmarked vial, I'm going to assume you reconstituted yourself from research pep tides. Please seek real medical advice from a real doctor. The good people of Reddit likely do not have medical or pharmacy degrees. Before you inject yourself with an unknown, unconfirmed substance, you should be 100% sure what it is and if it's safe."
Domestic News
Alabama pharmacists staged a walkout to support PBM reform. The FDA recalled contaminated medical products and a supplement made with undeclared prescription medicine.
Last week Alabama pharmacists staged a walkout in support of SB93, the Patient Access Bill, which would prohibit pharmacy benefit managers from reimbursing pharmacies less than the cost of a medication. Learn about the bad consequences of this practice.
The FDA posted recalls of three products:
- A lot of SinuCleanse soft tip squeeze bottle nasal wash system contaminated with a bacteria that could cause serious and life-threatening infections.
- Ten lots of a supplement made with undeclared tadalafil, which can harm patients taking prescription medicines that contain nitrates.
- Three lots of phenylephrine and sodium chloride made for hospitals by Central Admixture Pharmacy Services after the compounder’s raw material supplier reported black particulate matter in a sealed vial of phenylephrine.
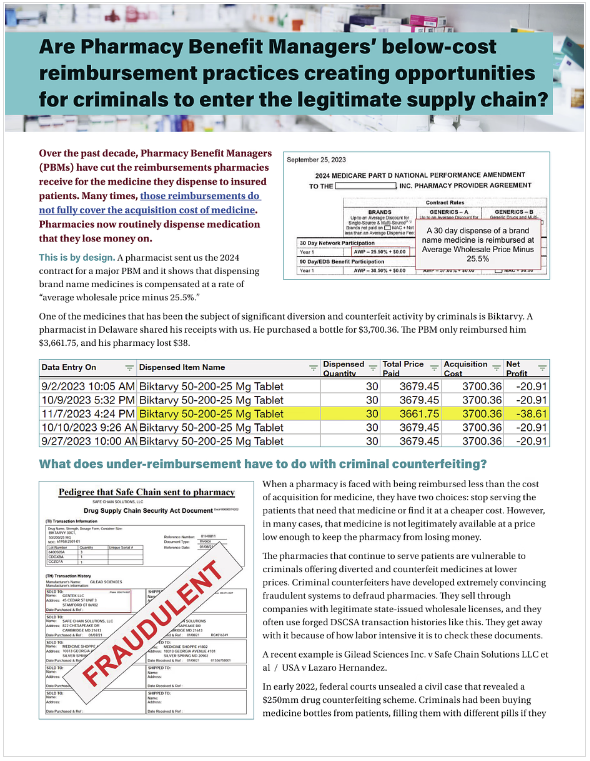
This 2-page summary explains the problems of PBM under reimbursements.
King County, Washington resident Braiden Wilson received an eight-year federal prison sentence for charges related to dealing narcotics on the dark web. Law enforcement who searched Wilson’s camper in 2024 found a large cache of weapons, kilogram quantities of fentanyl pills and a manual pill press.
Jarvis Jermaine Hampton of Meridian, Mississippi received a 220-month sentence for possession with intent to distribute methamphetamine after a search of his home in 2024 yielded methamphetamine, cocaine, a pill press and a machinegun.
International News
The RCMP shut down a pill press operation in Quebec.
The Royal Canadian Mounted Police dismantled a laboratory in Gore, Quebec, seizing several million pressed pills, a pill press and chemicals, precursors and excipients, including suspected methamphetamine.
Customs officers in Hong Kong arrested four individuals and seized HK$850,000 (US$110,000) in counterfeit medicines—including prescription treatments for skin conditions and reactive airway disease and traditional Chinese medicine—after a tip that a pharmacy was selling them
In Saudi Arabia, the Zakat, Tax, and Customs Authority seized 480,000 pregabalin pills and 37,000 Xanax pills as they were being smuggled into the country via King Abdulaziz Port and Al-Wadiah Border Crossing.