New initiative: Best practices for online pharmacy-to-pharmacy marketplaces
Today, PSM is announcing an effort to reduce sales of counterfeit and diverted medicines on online pharmacy-to-pharmacy marketplaces. Recent prosecutions have identified this problem, and after consultation with several platform owners and protection teams, we are proposing a set of best practices to make these platforms less hospitable to criminals selling unsafe medicine.
Read on for background on the issue, a description of our approach, proposal drafts, a project timeline, and instructions for how to get.
Background
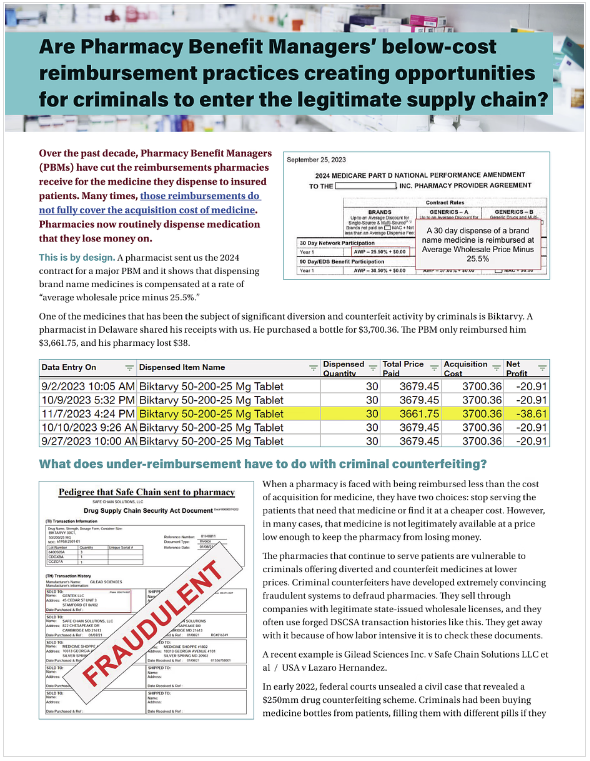
This 2-page summary explains the problems of PBM under-reimbursements.
Over half a dozen platforms exist to allow pharmacies to sell medicines directly to other pharmacies. Independent pharmacies near each other used to sell each other medicines to avoid temporary shortages that might inconvenience patients. This practice has become far less common for a variety of reasons including stricter wholesaling requirements, more just-in-time deliveries by distributors, prime wholesaler contractual obligations, and other factors.
However, the advent of the Internet allows anyone in the country to be a “neighboring pharmacy,” and a variety of online platforms have sprung up to allow sales of medicines between independent community pharmacies. Although reasons vary, pharmacies often sell on these platforms to recoup the cost of medicine that cannot be returned to the manufacturer/distributor for a full refund. They buy on these platforms to acquire medicines in shortage or to find better prices than available from their prime. The latter need has grown in response to systemic PBM under-reimbursements that threaten the survival of independents.
Safety
Products sold between pharmacies for specific named patients are exempt from the product tracing requirements of the Drug Supply Chain Security Act, and those exemptions are a draw for criminals and a threat to patient safety.
In USA v Boris Aminov (unsealed in 2023), for example, two individuals were indicted for selling $9 million of diverted medications, including $3.5 million in HIV medications, to unsuspecting pharmacies via unspecified online marketplaces.
PSM has ascertained that other online platforms such as Amazon and eBay have significant partnerships with pharmaceutical manufacturers to mitigate these risks. The manufacturers’ brand protection teams add value to the platforms by monitoring them for suspicious product listings. We are not aware of similar partnerships with existing online pharmacy-to-pharmacy marketplaces.
Read the indictment for USA v Boris Aminov
Our project and goals for 2025
The online marketplaces PSM are currently tracking are MatchRX, InStockRx, RxPost, Trxade, TradeNetRx, RxWorld, EzriRx and Rxeed. PSM would like to see them:
- Adopt practices that minimize the risk of counterfeit and diverted medicine being sold on their platforms; and
- Develop partnerships with brand protection teams and law enforcement to allow them to identify suspicious sales.
After discussions with several marketplaces and brand protection teams, we have developed a draft set of best practices we believe would make the platforms safer. We intend to:
- Publish these in draft,
- Gather and publish feedback and
- Publish a final set of best practices with changes based upon that feedback.
We will give the platforms several months to implement the changes and then conduct an evaluation of each platform’s compliance with the best practices.
Timeline
(subject to change)
- Until mid-May: gather comments
- Mid-June: publish de-identified comments
- Late June: publish final set of best practices
- September: begin evaluation of platforms
A note on gathering feedback and publishing comments
As we conduct calls and gather feedback from stakeholders, we will collect and de-identify comments before we re-share them. However, to assist the reader, we will identify comments by sector as long as that sector does not identify the commenter. Here are examples of the sectors we are planning to use, subject to change, to maintain anonymity:
- Marketplaces: Online marketplaces
- Pharmacies: Pharmacies and their advocates / trade associations
- Distributors: Distributors and their advocates / trade associations
- Manufacturers: Manufacturers, brand protection teams and their advocates / trade associations
- Regulators: Regulators, law enforcement, and their advocates / trade associations
- Patients: Safety and shortage advocates
Materials and how to comment
Please download a copy of our draft guidelines. You can submit comments to shabbir@safemedicines.org and eva@safemedicines.org. You can also request a meeting with us if you just want to talk through the topic with us. Drop us an email and we’ll send you a scheduling link.