4e Brands North America Issues Nationwide Voluntary Recall of Hand Sanitizer Due to Potential Presence of Undeclared Methanol (Wood Alcohol)
Update: On July 27, the FDA announced that 4e Brands was recalling all lots of their hand sanitizers due to their containing methanol. Read the updated alert here.
July 13, 2020
This is a reprint of an FDA Alert.
When a company announces a recall, market withdrawal, or safety alert, the FDA posts the company’s announcement as a public service. FDA does not endorse either the product or the company.
Company Contact Information
Consumers:
4e Brands North America LLC
888-843-0254
4EBrands8797@stericycle.com
Company Announcement
San Antonio, Texas, 4e Brands North America is voluntarily recalling ten (10) bottle sizes of Hand Sanitizers to the consumer level. The products are being recalled due to the potential presence of methanol (wood alcohol).
Risk Statement: Substantial methanol exposure could result in nausea, vomiting, headache, blurred vision, permanent blindness, seizures, coma, permanent damage to the nervous system or death. Although all persons using these products on their hands are at risk, young children who accidentally ingest these products and adolescents and adults who drink these products as an alcohol (ethanol) substitute, are most at risk for methanol poisoning. To date, 4e Brands North America has not received reports of adverse events related to this recall.
Recalled Products
These products are used as hand sanitizers and marketed to help decrease bacteria on the skin when soap and water are not available. The affected Hand Sanitizers are packaged in clear plastic bottles. The recalled products are as follows (a full listing of lot numbers in numerical order is included below the chart):
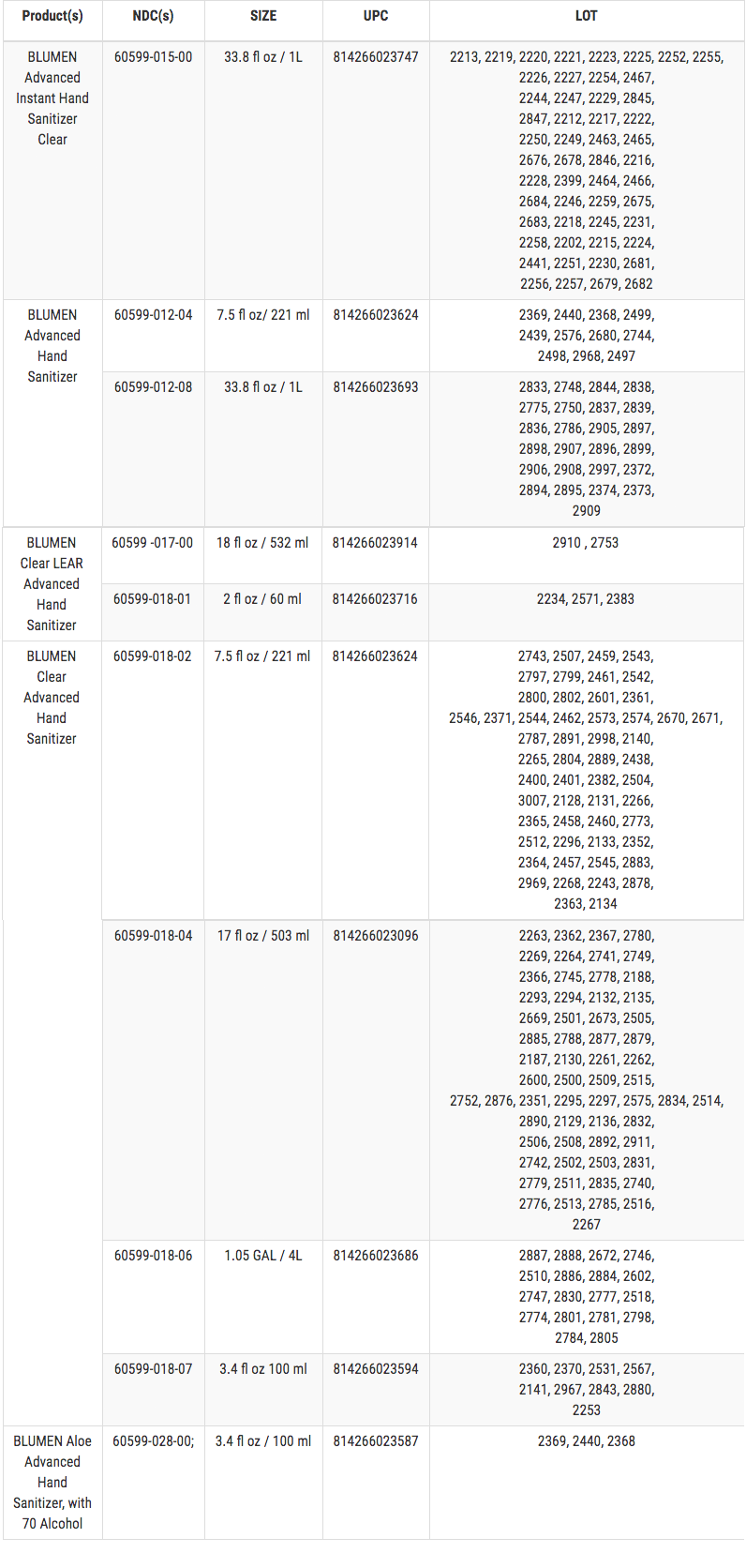
LOT NUMBERS

The Hand Sanitizer is in a clear bottle with a blue cap. The product label contains only blue, white, silver, and red coloring. The lot number is four digits and printed on the bottle.
The product was distributed nationwide in the United States through retailers and distributors.
4e Brands North America is notifying its distributors and retailers by recall letter and consumers via this press release. 4e Brands North America is arranging for the return and refund of all recalled products.
Consumers/distributors/retailers that have the product subject to this recall should stop using/distributing/selling Hand Sanitizer and return it to the place of purchase.
Consumers with questions regarding this recall can contact 4e Brands North America LLC during business hours: Monday – Friday 08:00 am – 5:00 pm EST at:
Toll Free: 888-843-0254
FAX: 888-214-7430
Email: 4EBrands8797@stericycle.com Event 8797
Consumers should contact their physician or healthcare provider if they experienced any problems that may be related to taking or using this product.
Adverse reactions or quality problems experienced with the use of this product may be reported to the FDA’s MedWatch Adverse Event Reporting program either online, by regular mail or by fax.
- Complete and submit the report Online
- Regular Mail or Fax: Download form or call 1- 800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178
This recall is being conducted with the knowledge of the U.S. Food and Drug Administration.
Below is a selection of the product images. To see all of the recalled products for this alert, click here.







