August 5, 2020 video: Behind the Scam: Uplabeling
This week's video goes “behind the scam” to show you “Uplabeling,” which is a technique that counterfeit criminals have used in the past to make major profits. In uplabeling, counterfeiters took a low-dose medical product and made it look like a more expensive, high-dose version of the same drug simply by changing the label.
How uplabeling works
A criminal finds someone within the supply chain who is willing to sell them vials of a drug that can be up-labeled to make a profit. Once purchased, the counterfeiting ring removes the labels from the low-dose vials and replaces them with fake labels for the more expensive high-dose product. The newly labelled medicine looks real, and it will pass basic testing: there’s real medicine in the vials, just not enough to be therapeutically effective. That’s usually sufficient because testing to be sure medicine is as strong as it claims to be is very expensive.*
No legitimate wholesaler would purchase vials of $400 medicine being sold for $50. A discount that big is a red flag that a product is counterfeit. However, if the medicine is sold and resold between small wholesalers for a little more each time, eventually the price will look like a reasonable sale rather than a shady transaction. As an extra bonus, if the vials pass through enough hands, it is impossible to trace them back to the manufacturer and they can get slipped back into the secure drug supply chain with no one the wiser.
The Drug Supply Chain Security Act (DSCSA)
As we’ll learn next week, this scam caused a lot of human suffering after counterfeiters sold 97,000 uplabeled vials of medicine that reached cancer and organ transplant patients across the U.S.
It also led to substantial reforms in the secondary drug wholesale market and, ultimately, to the Drug Supply Chain Security Act of 2013 (DSCSA). The DSCSA created nationwide licensing requirements for companies in the prescription drug industry and created a standards for an electronic track and trace system for prescription drug sales. When all the provisions of the act are in place, every company in the pharmaceutical supply chain from manufacturer to pharmacist will only be able to work with other licensed companies. Furthermore, every package of U.S. regulated prescription medicine will have a secure electronic record which lists every entity involved from the moment it was manufactured until the moment it reaches a patient.
These reforms make it substantially more difficult to uplabel medicine and slip it back into the legitimate supply chain.
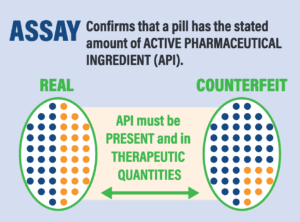
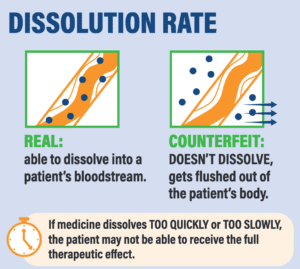
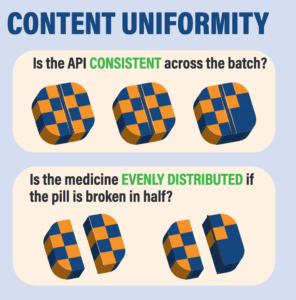
*A recent paper by Dr. Kristina Acri née Lybecker estimated that safety-testing medicine made or distributed outside the regulated U.S. drug supply chain would cost more than $2700 per single dose. The column at right shows tests that would be necessary.
Four Tests for Drug Safety
Sources for this week's video and blog
- “Drug Supply Chain Security Act (DSCSA),” U.S. Food and Drug Administration, last updated May 8, 2020.
- Sick Crime: Counterfeit Drugs in the United States, Subcommittee on Criminal Justice, Drug Policy, and Human Resources of the Committee in Government Reform, House Of Representatives, November 1, 2005.
- First Interim Report of the Seventeenth Statewide Grand Jury, Supreme Court of the State of Florida, Case No: SC02-2645, February 2003.
- Dr. Kristina M.L. Acri née Lybecker,“State Pharmaceutical Importation Programmes: An Analysis of the Cost-effectiveness,” Journal of Pharmaceutical Services Research, June 2020.
- Adam Fein and Dirk Rodgers, “State Drug Importation Laws Undermine the Process That Keeps Our Supply Chain Safe,” Stat, July 11, 2019.
How Do You Test Whether a Medication is Legitimate?, The Partnership for Safe Medicines, October 2019.