Prescription Drug Affordability Board Activity through October 18, 2024
Activities Summary
Colorado reviewed proposed changes to House Bill 23-1225 and Senate Bill 24-203 based upon months of stakeholder testimony and board review. However, the board voted to continue working on the rule making at their December 6th meeting. Additionally, the Colorado PDAB was instructed in Medicare’s Maximum Fair Price determination by PORTAL.
Maryland held no meetings in this period. Their next PDAB meeting will be October 28, 2024.
At Oregon’s October 2nd, 2024 PDAB meeting, Oregon’s PDAB consultants, Myers and Stauffer, provided a draft constituent group engagement report that summarized the constituent group recommendations. They also presented a hypothetical upper payment limit model analyzing potential 2023 savings based upon Medicare’s Maximum Fair Price. The PDAB staff presented policy recommendations, which were discussed but not approved by the board. At the state’s October 18, 2024 PDAB meeting the Department of Consumer and Business Services presented aggregate data collected from PBMs doing business in Oregon. The board discussed the Senate Bill 192 upper payment limit draft report and policy recommendations for the Oregon legislature. The board did not vote on these recommendations.
Washington will have its next PDAB meeting on November 13, 2024.
Activity by State
Meeting of the Prescription Drug Affordability Board (PDAB), October 18, 2024
Minutes of the September 6, 2024 meeting were approved.
Rule Introduction
Prescription Drug Affordability Board Rule 3 CCR 702:9 Part 1 - General Provisions and Part 3 - Affordability Reviews of Prescription Drugs (Redline with comments after letter here.)
Rule presented by Lila Cummings, proposed changes to House bill 23-1225 and Senate Bill 24-203. Input has been received over many months from stakeholders, interested parties, and written and oral commenters. Staff recommended that the rule making continue into the December 6th meeting, and plans to meet with the Rare Disease Advisory Council to incorporate their input into the rule.
Proposed Changes
- Identifying drugs for affordability review
- Law changed criteria for identifying drugs. Different criteria for types of drugs (biosimilar, brand name and generic)
- Any drug that meets a number of criteria might qualify. New criteria:
- Wholesale acquisition cost (WAC) of $,3000 or more per unit
- An increase of $300 or more in WAC/unit in the preceding 12 months
- Increase of 200% or more in WAC/unit in the preceding 12 months
- Treatments with a WAC of $30,000 or more
- Conducting affordability reviews
- Aligning rule with law that passed
- Staff to strike word annually
- Stakeholders asked for additional selection criteria, but because over 600 drugs are eligible, staff doesn’t recommend expanding eligibility criteria
- Board doesn’t have to look at every selection criteria for every drug
- Utilization of more than 12 individuals
- Board can ask for more data on drugs to be reviewed
- If drug is only a rare disease drug, board is required to get patient, stakeholder and Rare Disease Advisory Council input
- Board must ask stakeholders about viability of therapeutic alternatives
- Also assess price of drugs obtained through pharmaceutical patient assistance programs
- Changed timeline from 60 to 90 days
Oral Testimony
- PhRMA, Katelin Lucariello - Letter also provided.
- International Foundation for Autoimmune & Autoinflammatory Arthritis (AiArthritis), Tiffany Westrich-Robertson asked to provide raw data for patient feedback. Letter also provided.
- Coalition of State Rheumatology Organizations, Dr. Mila Kastsianok cautioned that not all therapeutic alternatives are therapeutically equivalent
- Advocates for Compassionate Therapy Now, Bridget Seritt said that federal law prohibits using “Quality-Adjusted Life Years (QALYs) in decision making process” in Section E, d(ii). Letter also provided.
- Amanda Boone, CF United: QALYS can not be used in the creation of an UPL. Letter also provided.
- Colorado Bioscience Association, Brian Warren was concerned that the board doesn’t have net price data to determine an individual's net price premium impacts. UPL is untested and may risk access. Letter also provided.
- Arthritis Foundation, Melissa Horn expressed concern that UPLs will impact patient care.
- Amy Goodman, Colorado BioScience Association. Letter also provided.
- Hope Stoner, Colorado Consumer Health Initiative was concerned about affordability of insurance premiums and asked to include patients who have not been prescribed a drug, but are eligible for it, in affordability reviews.
Extensive written testimony is found here.
Board Deliberation
Dr. Sami Diab, asked for an Executive Session to discuss QALYs in the rules and receive legal advice pursuant to section 24-6-402(3)a(iii), CRS and 10-16-1404(3), CRS. After the closed section, the board deliberated on adopting proposed updates to Rule Parts 1 and 3.
A member made a motion to continue rulemaking on December 6th, 2024 to allow additional stakeholder input and board deliberation. The vote passed.
- Discussion about revisions to PDAB Policy and Procedures: Policy No 4 (Affordability Review Policy and Procedure) Deliberations concerning unaffordability - No comments from board
- Upper Payment Limit topics from PDAAC Members
- PDAAC had questions about UPL (See image below)
- Proposed that PDAAC meet in December.
- PDAAC’s December meeting will include a review of candidates to fill vacancies on the council

- Upper Payment Limit Discussion with PORTAL
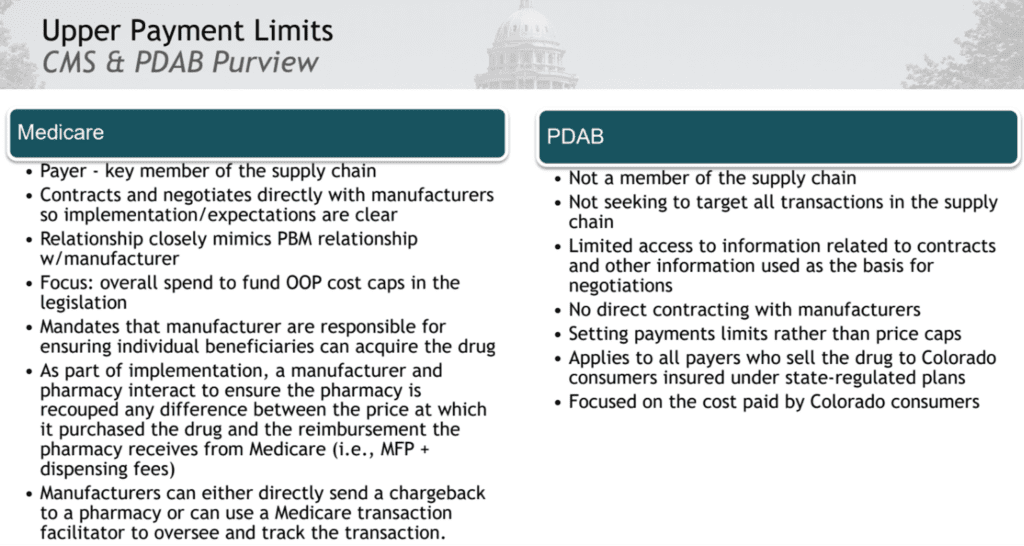
- Compared Medicare and PDABs. (See image below.)
- Differences impact the use of the Maximum Fair Price (MFP) for Medicare, because they directly negotiate with manufacturers. The PDAB can not.
- The PDAB can not impact the price of the drug, only the payment for that drug along specific parts of the supply chain.
- Medicare is a large national public payer. CO PDAB has a smaller population.

- Understanding Medicare’s MFPs - Overview of Medicare drug price negotiation (Slides available here)
- The board was instructed on the definitions and sources of the 12 factors to determine an UPL as required by 3 CCR 702-9 Part 4: Upper Payment Limits.
- Drug shortage list
- Impact to older adults and the disabled
- Wholesale acquisition cost
- Average sales price
- National average drug acquisition cost (NADAC)
- Out-of-pocket amounts
- Carrier paid amounts
- Retail discount amounts
- Public health care program fee schedules
- Estimates of manufacturer net-cost & net sales amounts
- Medicare’s maximum fair price
- Cost information voluntarily provided by wholesaler, pharmacists or provider
- The PDAB is limited to creating a UPL that can not be lower than statutory “best price.” “Best price” is equal to or lower than Medicare’s Maximum Fair Price. All Medicaid agencies get the “best price.” If they institute a UPL lower than “best price” they’ll be impacting prices across the country, which is illegal.
Public Comments
- Advocates for Compassionate Therapy Now, Bridget Seritt - “There’s a bias that could be coming from an insurance company or funding from Arnold Ventures. I would like to see that addressed just as thoroughly as the pharmaceutical bias has been addressed here as well. Letter also provided.
- CF United, Amanda Boone, CF United - Be aware that patients may be unwilling to speak up when they are judged for their disabilities by the costs of their medications.
- Healthcare Distribution Alliance, Leah Lindahl identified inaccuracies around wholesalers and how the process works. Will provide a letter addressing correction.
The next PDAB Meeting will be held on Friday, December 6, from 10 am - 2 pm MT.
Note: In 2023, the PDAB did prescription drug affordability reviews of Trikafta, Enbrel, Genvoya, Stelara and Cosentyx.
Maryland has two entities working together: the Prescription Drug Affordability Stakeholder Council (PDASC) and the Prescription Drug Affordability Board (PDAB).
The last meeting was September 10, 2024.
They are soliciting written comments informing the development of draft regulations and the cost review study process on Farxiga, Jardiance, Ozempic and Trulicity, and encouraging patient experience and clinician input. These are due on November 8, 2024. Comments should be submitted to comments.pdab@maryland.gov.
The next Prescription Drug Affordability Stakeholder Council Meeting will take place on November 4, 2024. More information can be found here.
The Prescription Drug Affordability Board held an Ad-Hoc meeting on October 28, 2024. More information can be found here.
The significant agenda items for the meeting:
- Opportunity for Public Comment
- Upper Payment Limit Action Plan- Update
- Cost Review Study Process- Update
- Administrative Update Chair’s Update
The meeting is not yet available to review online.
PDAB meeting, October 2, 2024
The Center for Evidence-Based Policy (CEBP) at Oregon Health and Science University (OHSU) made a presentation entitled “Oregon PDAB Support Options.” Previously, the board chair reported that though CEBP had asked all of the states workinging on PDABs to create a multi-state collaborative, this was not viable because the states were all on different trajectories. Oregon’s PDAB has been receiving assistance from the PORTAL team at Harvard for technical assistance provided by a grant through the National Academy of State Health Policy. CEBP offered options to support the PDAB, including providing stakeholder input, health process and systems engineering, data analysis and collection of evidence. CEBP is supported by two grants, one with Arnold Ventures and the other with The Commonwealth Fund.
Between April and July 2024, the Oregon Prescription Drug Affordability Board worked with consultants Myers and Stauffer LC to host 23 community meetings. The consultations completed an upper payment limit study required by statute and discussed savings possibilities for upper payment limits. The board chair, Ralph Magrish, quoted the following sources:
“The U.S. government's first-ever negotiated prices for prescription drugs are still on average more than double, and in some cases five times, what drugmakers have agreed to in four other high-income countries.” Beasley, Deena, “US will still pay at least twice as much after negotiating drug prices,” Reuters, September 3, 2024.
“Prices for these drugs after this negotiated maximum fair price are still 4.7 times higher than that of the United Kingdom’s National Health Service.” Mueller, Lucia, “Medicare negotiated drug prices 4.7 times higher than NHS negotiated prices,” PharmacyChecker.com, August 22, 2024.
“More than 50% of what Medicare spent on drugs from 2016 to 2018 was for drugs that were advertised on television.” Rosenthal, Elisabeth, “With TV drug ads, what you see is not necessarily what you get,” KFF Health News, September 17, 2024.
Magrish concluded that upper payment limits would impact medicine prices to consumers, premium increases, and bottom line profitability for small businesses and employees’ salaries.
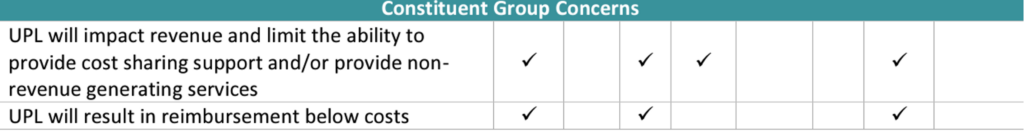
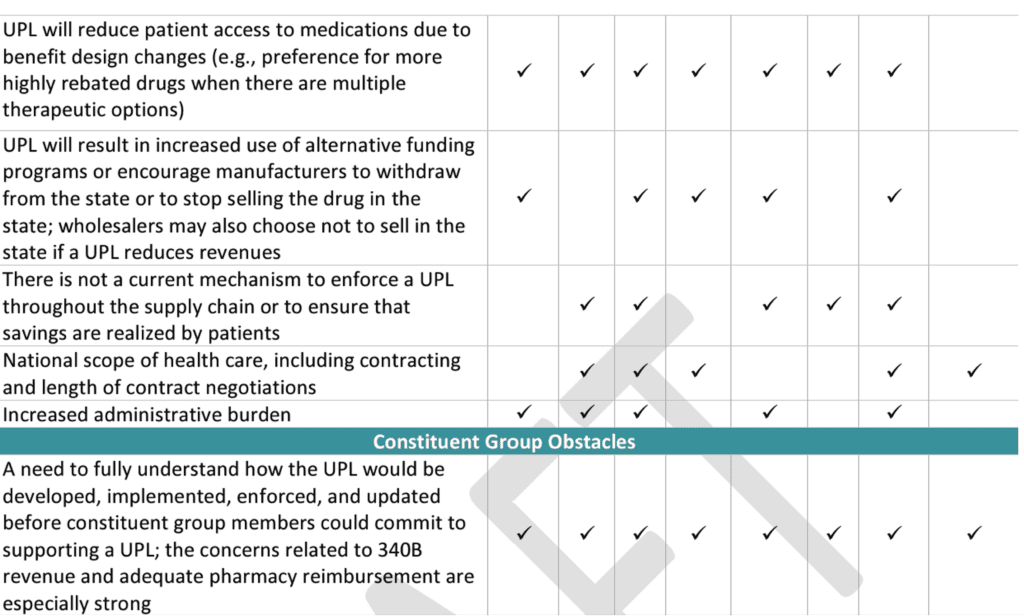
On August 21, Myers and Stauffer LC provided a draft constituent group engagement report with a summary table of constituent group recommendations:
They also provided a summary of the constituent group concerns and obstacles:
Details of the constituent feedback is available here.
Myers and Stauffer LC presented a hypothetical model of 2023 savings using Medicare’s maximum fair price (MFP). The consultants analyzed data collected from insurance carriers reporting drug prices, excluding Medicare, Medical, and others.
They also created three UPL scenarios for eight prescription drugs using monthly prescription and medical drug cost data from the Oregon Educators Benefit Board (OEBB) and Public Employees’ Benefit Board (PEBB) between April 2023 and March 2024.
- Under scenarios where there are no rebates due to an implemented upper payment limit, costs are projected to increase $8.9M or decrease by $10.7M (range of +1% to -1.1%).
- Under scenarios where 25%-50% of rebates are retained, the PEBB program could increase by $3.6M or save $20M depending on the upper payment limit selected. For the OEBB, the program is modeled to decrease by $.2M to $13.8M, based upon the upper payment limit selected.
Magrish exhorted the board to understand that an upper limit process will require a comprehensive, detailed plan for individual drugs.
The board and consultant discussed approaches that other states have leveraged to set upper payment limits.
- Net Cost - establishing a upper payment limit at or near existing average net drug price after all rebates & discounts
- Reference Pricing to Existing Benchmarks - Establish upper payment limit based on prices already negotiated or set by other entities
- Reference Pricing to Therapeutic Alternatives - Establish upper payment limited based on the price of drugs that can be substitute for the drug under consideration
- Launch Price Indexing - Establish an upper payment limit that uses product launch price and indexes price to the CPI.
- Percentage off of WAC - Establish upper payment limit as a fixed percentage off of WAC (For brand drugs, Federal Medicare rebate is 23% of AMP).
- Payer Return on Investment - “For a drug that has been subject to valid pharmacoeconomic research on value/cost savings, establish an initial UPL with a minimal lower costs and assess health plan savings over a set period.”
- Budget Impact-Based - Establish upper payment limit so that drug spending does not exceed a percentage of a budget.
- 340B Program Specific - Establish reimbursement adjustment for some of or all of the 340B entities.
Board discussion on the options was varied, with concerns about upper payment limits, rebate models, net up front costs, and negotiated prices from the Department of Veterans Affairs. Concerns were brought up that international pricing comparisons are not comparable. The process is complicated by the diversity and use of drugs, i.e. comparing a long-term generic drug to a new medication for the same disease. The Executive Director suggested that all of the methods may be used for different medications. Board members raised concerns that pharmacists may have difficulty implementing a complex process.
Asked what options are problematic, the board members replied:
- 340B Program Specific - Not helpful to the state as a whole or consumers.
- Budget Impact-Based - “Not sure it will get us where we need to be.”
- Launch Price Indexing & Percentage off of WAC - List prices can be externally increased which will make the UPLs higher.
The PDAB staff presented policy recommendations for the board’s approval. More details on these and the suggested recommendations below are found in the meeting document.
- Propose a language change from “nine drugs a year” for affordability reviews to “up to nine” drugs a year.
- Remove requirement that Department of Consumer and Business Services (DCBS) provide Prescription Drug Affordability Board (PDAB) with a list of prescription drugs each calendar quarter.
- Remove the generic drug report annual requirement, with a new provision that relevant content would be incorporated into the affordability review report. The information could include generics or biosimilar availability, pricing, and marketplace commentary when relevant to drugs under review.
The following additional suggestions were discussed, but not approved by the board.
- Expand prescription assistance programs (PAP) requirements to include manufacturer coupons and any other payment that reduces a patient’s out-of-pocket cost to fill a prescription. The board also recommended manufacturers be required to report on all patient assistant programs they maintain or fund.
- Implement mandatory reporting on copay accumulator and maximizer programs to ensure equitable access to essential medications and prioritize transparency.
- Require that all claims for prescription drugs and services provided by critical access pharmacies (CAPs), whether under Fee-For-Service (FFS) Medicaid, coordinated care organizations (CCOs), commercial insurance, or any prescriptions adjudicated through exchange payors, be reimbursed at the exact same rate as the CAP FFS Medicaid rate. This would ensure payment parity for all payors when reimbursing CAPs.
- Require any broker or entity facilitating the purchase of health insurance or prescription drug benefits for purchasing entities to provide an annual disclosure of all direct and indirect compensation received, as required by the CAA. The disclosure must include any commissions, fees, or other forms of compensation related to the transaction.
- Require all payors, including CCOs, commercial health plans, exchange-based health insurance plans, and PBMs operating within the state, reimburse pharmacies at a rate that is no less than the average actual acquisition cost (AAAC) of the drug plus the state determined dispensing fee.
- Any broker or entity facilitating the purchase of health insurance or prescription drug benefits for purchasing entities must provide an annual disclosure of all direct and indirect compensation received.
- Minimize reimbursement for all prescriptions: All payors, including CCOs, commercial health plans, exchange-based health insurance plans, and PBMs operating within the state, shall reimburse pharmacies at a rate that is no less than the average actual acquisition cost (AAAC) of the drug plus the state determined dispensing fee.
- Leverage collective buying power of state agencies.
- OHP FFS has a uniform prescription drug list (PDL) for some classes of prescription drugs. Extend the current PDL to all classes of prescription drugs to make sure providers are using the most cost-effective medications and to reduce their administrative burdens.
Written comments were provided by:
- Health HIV
- Mark Sturbois
- American Diabetes Association
- National Multiple Sclerosis Society
- PhRMA
Oral comments were provided by:
- Daria McGrew, PhRMA
- Board members
PDAB meeting, October 16, 2024
Executive Director Ralph Magrish provided a program update. Then, the Department of Consumer and Business Services presented aggregate data collected from PBMs doing business in Oregon. The board discussed the Senate Bill 192 upper payment limit draft report as well as policy recommendations for the Oregon legislature. All meeting materials are available here.
The Annual Drug Price Transparency Public Hearing will be Wednesday, December 4, 2024.
In his introduction to the next topic, Ralph Magrish reminded the board that in December of 2023, they recommended to the legislature, “Transparency loss can impact corporate behavior but the effect is often attenuated. However, minimal public information about PBM business practices and pricing models make state regulation of PBMs difficult. Collecting additional data could generate more insight into the role of PBMs in drug pricing and support the development of more substantive policy recommendations concerning PBMs in the future. So to that point I’d like to think that the legislature heard us when it adopted that particular piece of House Bill 4149 last year that directed the Department of Consumer and Business Services to begin collecting the following information from PBMs beginning in 2025. And those additions were the total dispensing fees paid to the PBM and to the pharmacies, the total administrative fees obtained and retained from manufacturers and carriers and money obtained through spread pricing, pay for performance, and similar means.
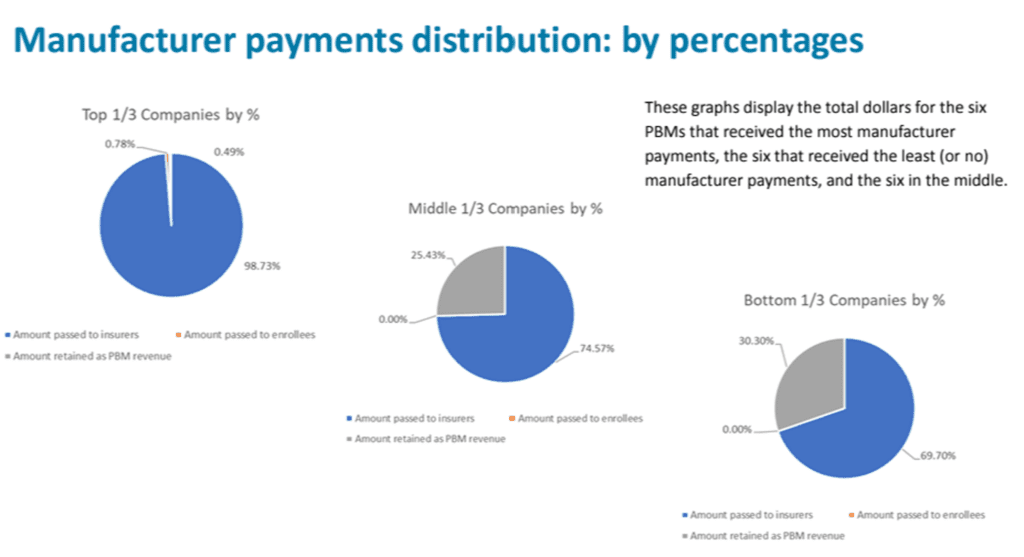
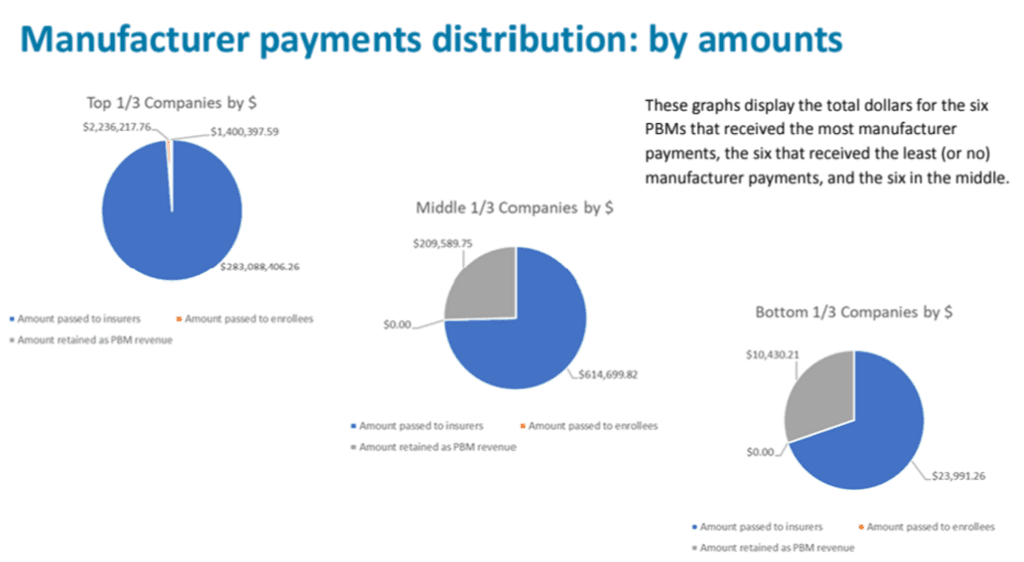
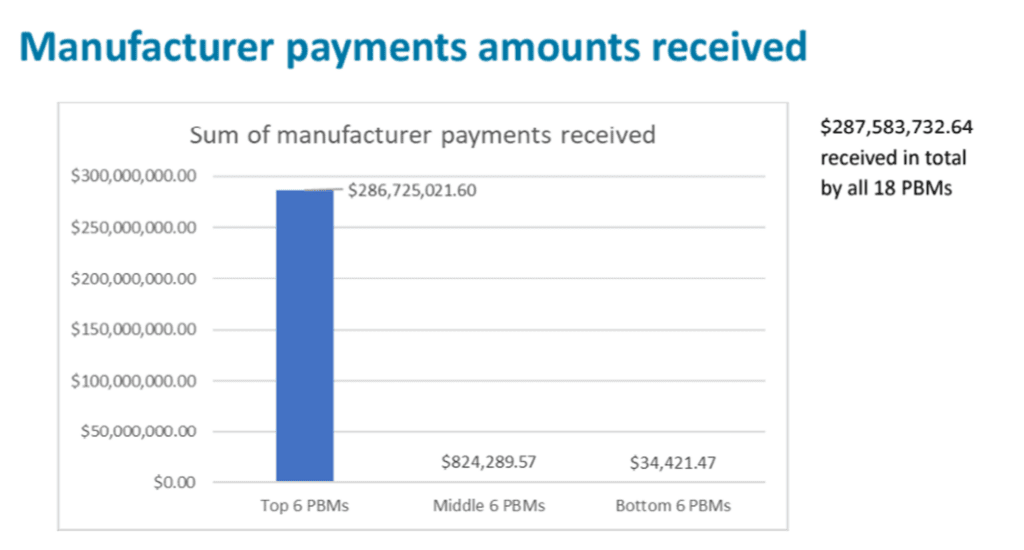
As required by the Senate Bill 192 (2023), the Drug Price Transparency program solicited 2023 data from 59 PBMs in Oregon on four topics: (18 PBMs submitted data; 41 were exempt from reporting due to being ERISA plans, workers’ compensation plans, Medicare or Medicaid programs or because they did not cover Oregon patients.)
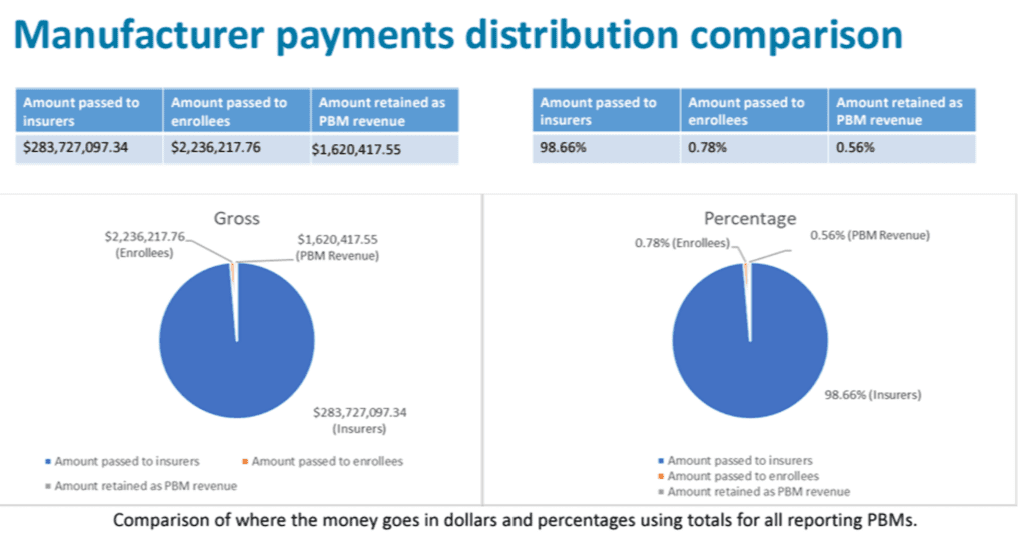
- Total manufacturer payments, fees, price protection payments, and any other payments from manufacturers related to managing benefits for insurers issuing health benefit plans in Oregon (i.e. rebates)
- Amount passed to insurers issuing health benefit plans in Oregon
- Amount passed to enrollees of health benefit plans in Oregon
- Amount retained by the PBM as revenue
The companies that responded:
- A&A Drug Co, dba Sav-RX Prescription Services
- AffirmedRx PBC
- Capital Rx, Inc.
- Caremark/PCS Health, LLC
- Cigna Health and Life Insurance Company
- Cigna Health and Life Insurance Company for TPM (Timber Products Manufacturers) Trust
- Drexi Inc.
- Express Scripts Administrators, LLC
- FairScript, LLC
- Magellan Rx Management, LLC
- Mark Cuban Cost Plus Benefits LLC
- Medimpact Healthcare Systems, Inc.
- Mitchell International, Inc.
- Navitus Health Solutions, LLC
- OptumRx, Inc.
- Prescryptive Health, Inc.
- Prime Therapeutics LLC
- True RX Management Services, Inc.
No data could be identified by PBM, the data can only be shown in aggregate.
The data showed that smaller PBMs retained higher percentages of their manufacturer rebates as revenue. The top six companies are working with hundreds of millions of dollars of rebates, while the other two tiers are working with multiples less.
Lessons learned:
- 41 of 59 PBMs were exempt from reporting requirements. The board wants more authority to collect information from the 41 PBMs that are currently exempt.
- Data could not be validated independently.
As of 2025, DCBS will be collecting additional data:
- The total dispensing fees paid to the PBM and to pharmacies
- The total administrative fees obtained and retained from manufacturers and carriers
- Moneys obtained through spread pricing, pay-for-performance or similar means.
The team believes the data is insufficient for policy analysis and wants to collect more detailed information in 2025. The board has many questions about the data because the statute prevented them from collecting details that would make sense of it.
The board continued to review and provide feedback to the UPL draft board report from the previous meeting.
A redline version will be made available to the board. Global comments from the board:
- How does this help the state save money?
- UPLs may help identify the tools that can be used in future years
Comments on the public engagement section:
- Takeaway: “be thoughtful as you proceed…You need to be very, very thorough and very cautious….This is really complex and groundbreaking.”
- A board member questioned the number of physicians responding to the public engagement.
- A board member recommended an advisory board of constituents.
Comments on the summary of states with PDABs:
- A board member asked if the board was communicating with the other states with PDABs. The executive director said that he freely shares information and communicates regularly with executive directors in other states, and that legislation allows data sharing.
Comments on the plan for establishing Oregon-specific UPLs
- A board member said they are attempting to make things as flexible as possible so that not all methods will be applied to all drugs.
- A board member asked about the impact of a UPL on stakeholders, including patient groups.
- A board member asked if they could consider evaluating the value of drugs and cost value.
- A board member asked for a rubric that would be consistent across UPLs to improve access or affordability. Need to establish what the criteria are for an ROI and WAC discount model.
- A board member wanted more clarity on the complexity of the UPL report. They commented that a lot more work would be needed to show what the effects would be.
- How does the board determine how a drug is unaffordable?
- Are there alternatives to UPLs? Is there a pass-through savings model that could be used instead? WV, KY and CA have them for public employees. (The statute requires the development of UPLs. However this could be a policy recommendation to the legislature.)
- Board members asked how this works with 340b institutions, which will lose the pricing spread they use to fund their missions. This area needs clarity. This should be removed from the UPL table, and put it in as a policy suggestion.
Comments about enforcement and authorities necessary for enforcement and about UPL implementation
- No comments.
Potential Senate Bill 844 clean up
- Propose language change from “9 drugs a year” for up to “ up to 9” drugs a year for affordability reviews
- Remove requirements that DCBS provide the PDAB with a list of prescription drugs each quarter. This information is based on the current year, and may not apply to the reporting requirement to review drugs from the previous year.
- Remove the generic drug report annual requirement, with a new provision that relevant content would be incorporated into the affordability review report. The information could include generics or biosimilar availability, pricing and marketplace commentary when relevant to drugs under review.
- Patient assistant program (PAP) reporting to the drug price transparency program (DPT) - expand PAP requirements to include manufacturer coupons and other payments that reduce a patient’s out-of-pocket cost to fill a prescription. Board also recommends manufacturers be required to report on all PAPs they maintain or fund.
- PBM and insurer reporting on copay accumulators and maximizers - implement mandatory report on copay accumulator and maximizer programs to ensure equitable access to essential medications and prioritize transparency. Board asked that copay accumulators may have been disallowed in HB 4113. Board concerned there is an ERISA issue. (Saved for next meeting’s discussion.)
- Uniform reimbursement rate for critical access pharmacies (CAPs). This applies to all PBMs that Consolidated Appropriations Act (CAA) disclosures about reimbursements and fees to employer plans with brokers. (Saved for next meeting’s discussion.)
- Minimum dispensing fees across all payers. (Saved for next meeting’s discussion.)
- Minimum dispensing fees for all prescriptions.
- Prohibition of below-cost reimbursements.
The board discussed how to reach a conclusion on these items, consensus or vote. No decision was reached. Votes will be done on individual items.
- Community Access National Network, Rainier Simons, Director of State Policy
- HIV+Hepatitis Policy Institute, Carl E. Schmid II, Executive Director
- Johnson & Johnson, Blasine Penkowski, Chief Strategic Customer Officer
- PhRMA, Dharia McGrew, Director State Policy
- Partnership to Improve Patient Care, Tony Coelho, Chairman
- Regence Blue Cross Blue Shield, Mary Anne Cooper, Director of Government Relations
4 oral comments
- Johnson & Johnson, Terrell Sweat, Director of State Government Affairs
- PhRMA,Daria McGrew, Director State Policy
- Lauren Sandt, Executive Director, Caring Ambassadors Program
- John Covello, Independent Pharmacy Cooperative
The next meeting will be November 20, 2024.
Washington’s PDAB did not meet in October. The last meeting was September 18, 2024. The next meeting will be on November 13, 2024.
Standout resources and coverage
"Prescription Drug Affordability Boards (PDABs): What are they and why should advocates care about them?" by Healthcare in Motion: Prescription drug access and pricing have been in the policy spotlight at the federal level over the past few years, with Congress taking unprecedentsteps to respond to high prescription drug pricesin Medicare through passage of the Inflation Reduction Act (IRA) in 2022. But state governments are also finding ways to address the rising costs of drugs. One mechanism states are using to evaluate and curb the prices of certain high-cost drugs are Prescription Drug Affordability Boards(PDABs). A PDAB is an independent board made up of experts tasked with analyzing—and in some cases, acting to reduce—high drug prices in the state. Read on to learn more about PDABs and how their decisions may impact individuals who rely on high-cost medications to stay healthy.
"What you need to know: Prescription Drug Affordability Boards and people with CF" by the Cystic Fibrosis Foundation: More states are establishing prescription drug affordability boards to assess and address the cost and affordability of prescription drugs. Learn more about what these reviews mean for you and your loved ones.