Prescription Drug Affordability Board Activity through November 15, 2024
Activities Summary
Colorado held no meeting in November. The next meeting will be December 6, 2024.
Maryland's Prescription Drug Affordability Board (PDAB) met on October 28, 2024 and the Prescription Drug Affordability Stakeholder Council (PDASC) met on November 4, 2024. Both bodies are working together to develop an Upper Payment Limit Action Plan. The PDAB staff is developing a dossier to make affordability determinations while the board is seeking guidance on the impact of the prescription drug cost relative to insurance benefit design. Staff are collecting additional data about rebates between insurance companies and manufacturers, cost and utilization data from state Medicaid and state employees, and out-of-pocket costs. The board is using the proposed new review process to conduct a cost review on six drugs.: Farxiga (dapagliflozin), Jardiance (empagliflozin), Ozempic (semaglutide), and Trulicity (dulaglutide), as well as Dupixent (dupilumab) and Skyrizi (risankizumab), which have not yet had a request for information (RFI) completed. The next PDAB meeting is November 25, 2024.
The PDAB Executive Director presented to the PDASC on the upper payment limit and policy review process and cost review study process. The following discussion covered definitions and implementation of upper payment limits. The next PDASC meeting is scheduled for December 16, 2024.
Oregon’s ’s next meeting is November 20, 2024.
A recording of Washington’s November 13, 2023 meeting is not yet publicly available.
Activity by State
The next PDAB Meeting will be held on Friday, December 6, from 10 am - 2 pm MT.
Maryland has two entities working together: the Prescription Drug Affordability Stakeholder Council (PDASC) and the Prescription Drug Affordability Board (PDAB).
PDAB Meeting October 28, 2024
The Prescription Drug Affordability Board held an Ad-Hoc meeting on October 28, 2024. Review the agenda and comments. Watch the meeting.
Opportunity for Public Comments
No oral comment.
Updates from Executive Director Andrew York:
The legislative policy committee needs to approve the action plan. The board and legislative policy committee staff have published a draft of the regulations on the website, and they are asking for public comment through Friday, November 8th. The stakeholder council will have the opportunity to weigh in on the draft regulations and provide comment.
The board is considering a monitoring plan for the Upper Payment Limit Action Plan, which requires publication. The board has two questions:
- What should they be monitoring for: shortages, access issues, or unintended consequences
- Should they also monitor for compliance with regulations?
For the monitoring plan, the board is considering outreach efforts to determine which drugs people are having the greatest difficulty accessing as well as surveying the national associations, hospitals and federal FDA shortage lists. (The state has data on shortages, and the Food and Drug Administration has data on national shortages.) Under the statute, the board is responsible for monitoring the availability of prescription drug products for which it sets an upper payment limit and to take action if there is a shortage of that product.
York also mentioned the addition of two new sections to the action plan: one on the reconsideration procedure and another on the hearing procedures. The reconsideration procedure is a complicated process that will be reviewed by the stakeholder council. He is interested in public feedback on the reconsideration process and the new hearing procedures that allow for public input.
The board is currently in the process of conducting a cost review on six drugs selected for the cost review study process. The board approved proposed regulations to update the cost review study process to account for the new policy review process. The preliminary determination step allows the board to use the policy review process on these drugs if they decide to move forward. The proposed regulations were published in the November 1st register and will be available for comments on December 2nd, 2024. The board can approve the final regulations 45 days after the proposed rule, and they will go live 10 days after the regular register publishes.
Staff is working on background analyses for four drugs, Farxiga (dapagliflozin), Jardiance (empagliflozin), Ozempic (semaglutide), and Trulicity (dulaglutide) and two additional drugs, Dupixent (dupilumab) and Skyrizi (risankizumab) that have not yet had an RFI completed. The cost review process has an automatic 60-day comment period, but the board is seeking more clinician and patient feedback. They have opened public comments for another two weeks, especially seeking written comments from public members and patients
Administrative Update Chair’s Update
The next Prescription Drug Affordability Board meeting will be held in-person November 25, 2024, at 2 PM. A December meeting may be added based on the progress made in November.
PDASC Meeting November 4, 2024
The PDASC met on November 4, 2024. Meeting materials are available here. Watch the meeting.
After calling the meeting to order and roll call, the minutes from the August 26, 2024 meeting were approved.
- No oral comments.
- Written comment was provided by Tiffany Westrich-Robertson of Ensuring Access through Collaborative Health (EACH) Coalition.
Update and Presentation available here for review
- UPL Status and Next Steps
Board approved UPL Action plan on 9/10/2024 - Legislative policy committee approved UPL Action plan on 10/22/2024
- Next step: Adopt regulations establishing Policy Review Process
Public feedback on draft regulations was requested on October 28, 2024 with comments closing on November 8, 2024.
The board would need to develop final regulations that it would then approve to be published. If published, they will go through the full notice and comment period according to the administrative procedure rules of Maryland. The purpose of the day’s presentation was to go over the regulations and gather general feedback on the best way to work through some of the processes.
- Amendment to COMAR14.01.01.01, and New Regulation 14.01.01.06 - Amendments were made to improve definitions and hearing procedures.
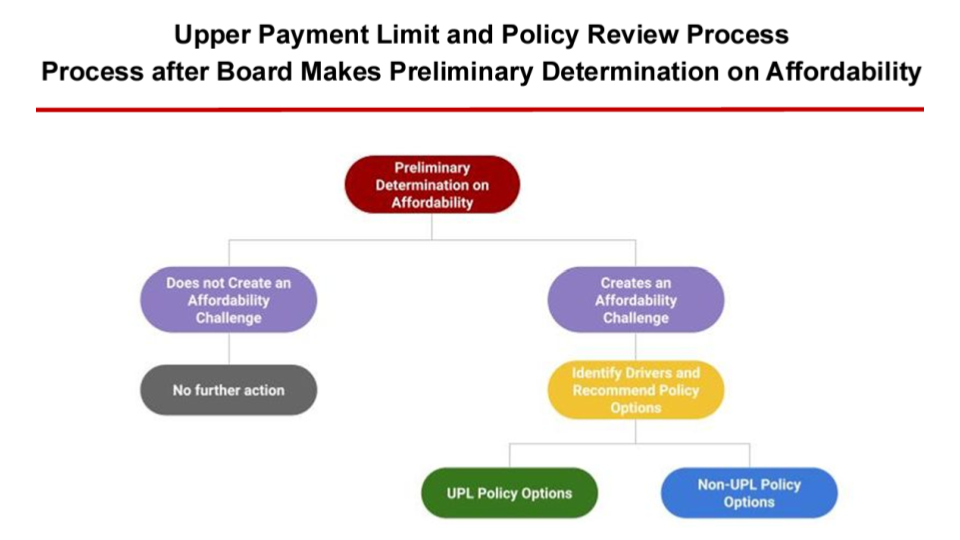
The following chart describes how the board will determine whether a drug will have an UPL applied to it. If the board does not cause an affordability challenge, there is no further action necessary, and the final cost review study will be published. If the board determines that a drug causes an affordability challenge, the board must identify policy reasons why a drug is unaffordable. Once that is determined they can make policy recommendations to address the cost drivers, which may include an upper payment limit action plan or other policy recommendations.
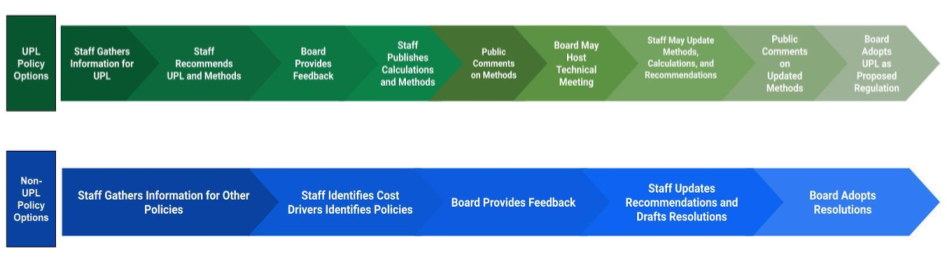
The options break into two big buckets, UPL and non-UPL policy options. The non-UPL policy options are recommendations to other state entities outside of the PDAB’s authority. The staff recommends methodologies to address cost drivers, which may vary depending on the drug being evaluated. If the drug is eligible for a UPL, staff will publicly publish the UPL calculation to gather technical feedback. Any changes made will be published for public comment. The final package will be provided to the board for adoption of the UPL as a proposed regulation.
- New Chapter - COMAR 14.01.05 (Policy Review, Final Action, Upper Payment Limits)
- Definitions specific to this process were created
- E.g., ‘“Upper payment limit” or “UPL” means the amount established by the Board that an eligible governmental entity may not exceed when purchasing or paying for the ingredient cost for a prescription drug product after all price concessions, discounts, and rebates.
- Other definitions not detailed here. The board was asked to read and comment on it.
- Criteria for setting upper payment limits: The Board shall:
- Consider the cost of administering the drug and delivering the drug to consumers, as well as other relevant administrative costs;
- Determine whether an upper payment limit is an appropriate tool to address the drivers of the affordability challenge identified for the prescription drug product;
- Set an upper payment limit in a way to minimize adverse outcomes and minimize the risk of unintended consequences; and
- Prioritize drugs that have a high proportion of out-of-pocket costs compared to the net cost of the drug.
- Criteria for setting upper payment limits. The Board shall NOT set an UPL if:
- Utilization of the prescription drug product by eligible governmental entities is minimal; or
- The prescription drug product is a generic and there are nine (9) or more marketed therapeutic equivalents for the product.
- The Board shall not set an upper payment limit at an amount that:
- Impacts statutory or regulatory amounts, such as Medicaid Best Price; or
- Is lower than the Medicare Maximum Fair Price.
- Policy review and final action process overview
- Information gathering
- Informational hearings
- Stakeholder council input
- Expert testimony hearings
- Board staff research
- Eligible governmental entities
- Preliminary policy recommendations
- Policy actions other than UPLS
- UPLS
- Process for establishing a UPL
- Staff recommends methodologies and contextual info
- 7 different potential methodologies (see presentation slide 21 for details)
- Contextual information for the medication (see presentation slide 22 for details)
- Upper payment limit values will be determined by board calculations and analyses using the methodology and contextual information provided by staff as well as public comment.
- The board may convene a technical input hearing.
- Revisions to conclusions may be made after the hearing.
- Final policy action
- Information gathering
- Establishing and monitoring an UPL - The board will adopt a regulation that sets the UPL and develop a monitoring program.
- Reconsiderations of an upper payment limit - The board has the authority to modify, suspend or repeal an UPL and conduct new cost review studies.
- Definitions specific to this process were created
Questions were fielded by members of PDASC.
The stakeholder council discussed the differences between informational and technical hearings procedures, focusing on time constraints, the hearing recording, and the chair's discretion. The council emphasized the importance of transparency and openness in future hearings and the need to consider public comments. Final recordings will be publicly available and accessible to ensure clarity.
Council member discussion and questions:
- They discussed the need for a definition of net ingredient cost, the Centers for Medicare and Medicaid Services (CMS) maximum fair price and the timeline for the regulation. Staff replied that there is no fixed timeline for setting upper payment limits. They are currently working through the process to see how long it takes to make estimates and recommendations.
- The definitions of net cost and minimal utilization are unclear, as they may include patient cost share or insured out-of-pocket member cost share. The definition of “minimal utilization” is ambiguous. Staff replied that setting a threshold for the upper payment limit for prescription drugs can be challenging due to the cost of the drug and the number of patients affected. The general principle is that if there is enough market competition, an upper payment limit is not necessary.
- Why exclude an UPL if there are nine or more generics, when the literature suggests a three-to-five range for maximum generic savings? Staff replied that the three-to-five range could be manipulated by manufacturers and that there is full saturation of generic competition.
- Does the UPL impact Medicaid's best price? Staff replied that the high-level principle suggests that the formulaic output should not be lower than the Medicaid best price. UPLs could potentially impact the Medicaid best price and the monitoring plan.
- Members expressed concerns about the lack of guidance in the draft regulations regarding enforcement mechanisms and monitoring procedures. Staff replied that that enforcement is a separate part of the statute and lives with the AG's office.
- When will cost relief for people struggling to afford medications happen?
- Why was “patient” excluded from the definition of purchaser? Does that exclude patients who are paying cash or have insurance plans? Staff responded that the working definition of “purchaser” is an eligible governmental entity.
- How will the regulations define ”affordability challenges?”High out-of-pocket costs for patients or challenges to the state healthcare system? The board articulated these concerns as part of their preliminary determination in the cost review study process.
- How do you address pharmacy and infusion centers’ concerns that the UPL may not cover the cost of administering and delivering drugs to consumers? The board does not intend for upper payment limits on eligible governmental entities to change reimbursement on the [patient] reimbursement side.
- If a drug is on the FDA shortage list, is it automatically suspended from the UPL? Yes, if it’s on the FDA shortage list. However, there is no automatic suspension in Maryland, as there is no specific trigger for shortages. Instead, a process is created to identify and flag shortages for the board, encouraging immediate action. The board can reconsider the UPL if they become aware of a shortage.
- How will the rebate pass-through process impact the UPL? The UPL is the final net cost to the purchaser including any rebates or discounts, using a framework like the Massachusetts and New York Medicaid programs.
The board is currently in the process of conducting a cost review on six drugs selected for the cost review study process. The board approved proposed regulations to update the cost review study process to account for the new policy review process. The preliminary determination step allows the board to use the policy review process on these drugs if they decide to move forward. The proposed regulations were published in the November 1st register and will be available for comments on December 2nd, 2024. The board can approve the final regulations 45 days after the proposed rule, and they will go live 10 days after the regular register publishes.
Staff is working on background analyses for four drugs, Farxiga (dapagliflozin), Jardiance (empagliflozin), Ozempic (semaglutide), and Trulicity (dulaglutide) and two additional drugs, Dupixent (dupilumab) and Skyrizi (risankizumab) that have not yet had an RFI completed. The cost review process has an automatic 60-day comment period, but the board is seeking more clinician and patient feedback. They have opened public comments for another two weeks, especially seeking written comments from public members and patients
Next Meeting Details
The next PDASC meeting will be Monday, December 16, 2024 at 2pm EST.
The next meeting is November 20, 2024.
Washington’s PDAB met on November 13, 2024. Agenda available here. Video not yet available for review.
Standout resources and coverage
Are PDABs Pushing the Limit? Drug Pricing Complexities Collide with Implementation, Morgan Lewis, October 4, 2024
"While Oregon has started questioning the practical benefits to PDABs setting UPLs, Maryland has conversely approved a process that could lead to UPLs on high-cost prescription drugs for state health plans."
"In Maryland, drug price controls won’t help patient affordability," Kelly Schulz in Maryland Matters, October 26, 2024
"At a time when Maryland could be accelerating medical innovation to meet the world’s health challenges, our state’s policymakers are taking steps that could curb medical advancements and disrupt patient access and care."