April 7, 2025: A study examines FDA-OCI's enforcement actions over a five-year period
Major Stories
A 2022 study examined the 130 enforcement actions done by the U.S. FDA-Office of Criminal Investigations from 2016 through 2021. The study found that 83 actions involved counterfeit medicine sold via the Internet and patients received counterfeit medicine in 109 actions when a legitimate prescription was not required.

You can read an abstract of the study here.
Domestic News
Lawsuits filed against two compounding pharmacies and three cases involving pill presses in the news.
Drugmaker Eli Lilly sued two compounding companies selling unapproved tirzepatide, the main ingredient in Mounjaro and Zepbound, with added vitamin B, a combination of products that has not been proven to be safe or effective. Last month a federal judge ruled that compounding pharmacies could not continue to sell copies of tirzepatide or semaglutide in the U.S. now that the drug shortage has been resolved.
A federal jury found Darren Tramaine Tony Mitchell of Vallejo, California guilty of drugs and firearm charges. Agents recovered methamphetamine-laced pills, an industrial pill press and firearms in his home after he negotiated a transaction for 1,000 methamphetamine pills with a buyer who testified at trial.
Law enforcement seized fentanyl, firearms and a pill press from a residence in Cane Ridge, Tennessee.
Charges were filed in federal court against a Georgia man after law enforcement seized a pill press and 240,000 fentanyl pills while conducting a search of his residence. Georgia: update to a previously tagged incident.
Image included in civil lawsuit filed by Eli Lilly again Strive Pharmacy
International News
Illegal injectable drugs seized in Canada, counterfeit Ozempic pens in Australia, and other counterfeit medicine news from India, Pakistan, Iceland, and Cuba.
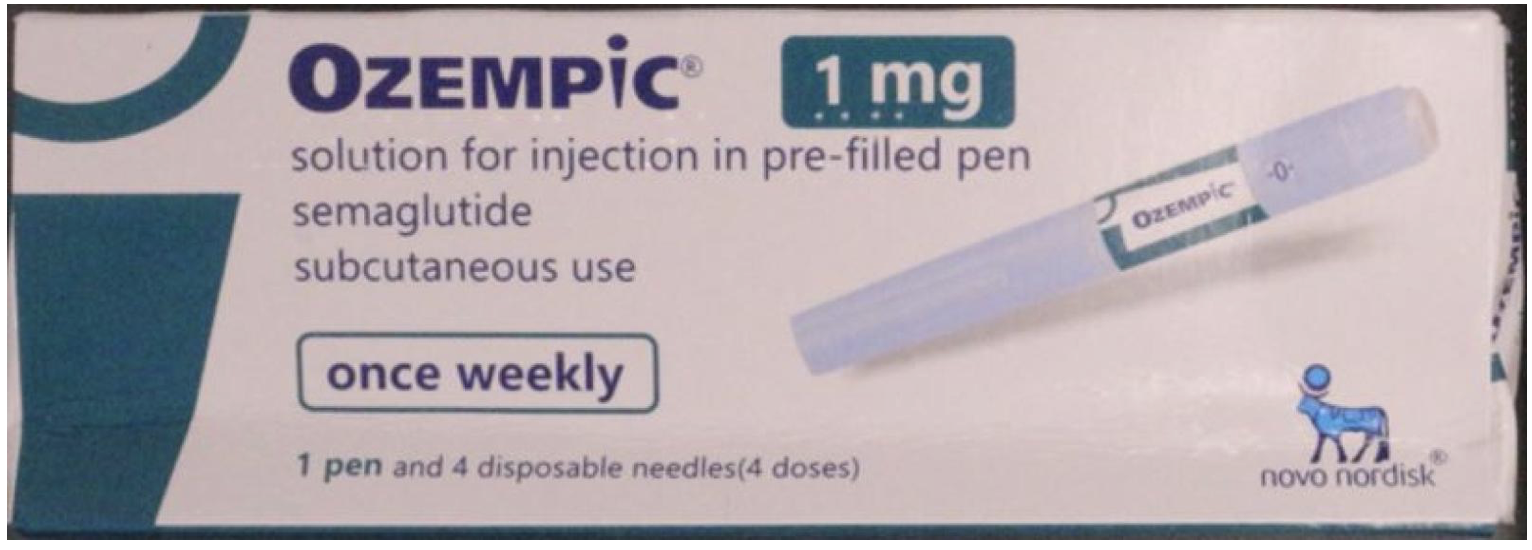
Image of counterfeit Ozempic box (Australia's TGA)
Health Canada seized more than two dozen different types of illegal, injectable peptide drugs from a Calgary business.
Australia’s Therapeutic Goods Administration announced that counterfeit Ozempic pens had been stopped at the Australian border. These are different from the relabeled insulin pens the agency warned about in 2024.
India’s Central Drug Standards Control Organisation (CDSCO) announced that tests identified 103 medicines as substandard, including a counterfeit version of Telma H, which treats high blood pressure.
The Drug Regulatory Authority of Pakistan arrested people selling unapproved versions of contrast dye used in medical imaging tests, and issued an alert about falsified propylene glycol adulterated with ethylene glycol (EG) in the Pakistani market. Hundreds of children in Cameroon, Gambia, India, Indonesia and Uzbekistan died after being treated with cough syrup contaminated with EG in 2022 and 2023.
Cuban regulators issued an alert about counterfeit allergy, congestion, epilepsy, and pain treatments.
Customs officers at Iceland’s Keflavík Airport announced the seizure of 20,000 counterfeit Oxycontin tablets made with nitazenes. This follows the recent WHO warning of the same type of counterfeit pill also containing a nitazine being reported in Switzerland.