Prescription Drug Affordability Board Activity through March 31, 2025
Activities Summary
Colorado: Colorado's PDAB cancelled its March meetings.
Maryland: Maryland's PDAB met on March 24, 2025 to begin a cost review of Farxiga and review feedback about updates to PDAB regulations.
Oregon: Board members met on March 19, 2025, where they elected a subset of drugs for affordability reviews. 31 people and organizations submitted public comment.
Washington: Washington's PDAB did not meet in March. The next meeting will be May 21, 2025.
Activity by State
Maryland PDAB meeting, March 24, 2025
Agenda | Recording | Meeting materials: Written comment packet; Cost Review: Presentation on process, Dossier on Farxiga, Sections 1-3; Regulations: Proposed Amendments, Comments on proposed regulations
After approval of the closed session minutes from January 27, 2025, public comment was submitted.
4 written comments from Community Access National Network, Ensuring Access through Collaborative Health (EACH) Coalition and Patient Inclusion Council (PIC), Let My Doctors Decide Action Network and Dr. Kate Sugarman were published to the website.
Dr. Olson, a physician practicing in Baltimore who supports cost reductions for diabetes medications, provided an oral comment. She frequently prescribes Jardiance and Farxiga, and worries that patients will buy medication from unsafe, foreign sources, or struggle with high co-payments. However, the veterans she treats receive the medication at lower prices and have better outcomes.
Staff Presentation – Cost Review Study Dossier, Sections 1-3 Overview (Farxiga)
Legislation states that the board may
- determine whether the use of a prescription drug will cause affordability changes to the state health system or high out-of-pocket costs to patients.
- determine whether the use of the drug that causes these costs is consistent with FDA approved use or standard medical practice.
- identify the circumstances where the drug may cause an affordability change to the state health system or high out-of-pocket costs to patients.
Six drugs have been selected for the cost review study process and information has been requested for Farxiga, Ozempic and Trulicity. The staff reviewed the information and the board must now make a preliminary determination per the three points above.
The staff has assembled a cost review dossier. The board will be discussing the first three areas today: background information, clinical information and regulatory approval. Each COMAR regulation is a different area, called an “element,” for review.
- Background information - Identifies drug under review
- Proprietary and non-proprietary name
- NDCS
- All previously identified
- Some are no longer active
- Clinical information – diseases & conditions treated
- COMAR 14.01.04.05C(1)(g)(i) – FDA indications, doses, and standard medical practice
- Staff reviewed FDA label and published clinical guidelines
- COMAR 14.01.04.05C(1)(g)(i) – FDA indications, doses, and standard medical practice
- COMAR 14.01.04.05.C(1)(g)(ii) – Disease burden of condition treated
- Impact of the conditions for the drug product
- Based on published literature review
- Regulatory approval and market context – history of approval drug use
- COMAR 14.01.04.05.C(1)(g)(ix) – analysis of approval process
- COMAR 14.01.04.05.C(1)(g)(x) – analysis of shortage status
- COMAR 14.01.04.05.C(1)(g)(xi) – market analysis including lifecycle & patient management, regulatory exclusivities and product hopping
This was followed by a closed session to consider and discuss confidential, trade secret and proprietary information, under GP § 3-305(b)(13) and HG 21-2c-03(e)(1)(iv) (Farxiga, Jardiance); and to consult with counsel and receive legal advice regarding proposed regulations, under GP § 3-305(b)(7).
This is a follow up to the working session in February's meeting. Staff received 135 pages of comments and will cover ones that weren’t covered in February.
A full presentation about comments and response from staff is available here. Comments fell into three categories:
- Definitions, lack of procedures on certain topics, and economic impact statement
- UPL procedures and policy options
- Legal concerns
Staff recommend these revisions:
- For clarity, replace term “methodology” with “framework” throughout.
- Clarify broad scope of designee by removing term “staff” in COMAR 14.01.01.06 hearing provisions.
- As discussed at last meeting, for purpose of clarity, staff recommends amending COMAR 14.01.05.05B(2)(d) (non-UPL) “possible implementation of the policy through legislation, regulation or enforcement.” And for the purpose of clarity and completeness, adding to COMAR 14.01.05.05C(2) (UPL) “strengths and weaknesses of the policy,” “potential impacts of the policy” and “possible implementation of the policy through legislation, regulation or enforcement”
The board approved the proposed amendments to COMAR 14.01.01.01 (Definitions); new COMAR 14.01.01.06 (Hearing Procedures); new COMAR 14.01.05 (Policy Review, Final Action, Upper Payment Limits).
Administrative Update
- Staff will submit regulations will be updated in the register.
- September's meeting has been moved to 9/15/2025. November meeting will be 11/17/2025. The May 19 meeting will be virtual.
- Two positions are open on PDAB's staff: policy analyst and administrative program manager.
New legislation has passed in Maryland: Senate Bill 357 and House Bill 424. The Lowering Prescription Drug Costs for All Marylanders Now Act adds members to the Stakeholder Council and requires the PDAB to confer with the Maryland Medical Assistant Program before setting payment limits. The bill adds representatives from the rare disease community, a patient advocacy organization and oncology. Under the bill, the board can reconsider payment limits for drugs that become part of a shortage but will not be able to establish a new payment limit for a drug that is currently in shortage.
The bill would also require the board to issue a report one year after setting a payment limit. The report would need to detail the impact of the limit on patient out-of-pocket costs, patient health insurance premiums, reimbursement rates for pharmacies and shortages or supply disruptions.
Senate Bill 406, related to workers compensation, may have implications for the PDAB.
The next meeting will be held May 21, 2025 at 9 a.m. EDT
Oregon PDAB meeting, March 19, 2025
Agenda and materials | Video | Public comments
After routine items, Executive Director Magrish gave a program update:
The legislature is in the process of changing the PDAB’s number of drugs to “up to nine drugs” rather than fixed at nine and changing timing on the generic drug report to annual from mid-year. Magrish spoke at the Oregon State Pharmacy Association’s mid-winter continuing education seminar in Lane County.
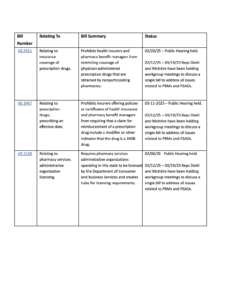
Review bill summaries with links to legislature pages.
31 organizations and individuals submitted written comments.
Three spoke at the meeting:
Dharia McGrew of PhRMA expressed appreciation for changes that will keep personal contact info confidential. She urged for more robust engagement from stakeholders and asked how proprietary business information will be kept confidential, how the data will be used in performing affordability reviews as well as for clarity in questions i.e. price concessions to plans via prices to PBMs and more detail in data dashboard dictionary.
Tiffany Westrich-Robertson of AiArthritis and Patient Inclusion Council thanked the board for considering redlines that PIC submitted and asked for revisions of definitions for clarity for patients.
John Mullin of the Oregon Coalition for Affordable Prescription Drugs expressed supports the PDAB. See letter in written comments for more details.
Request for Information forms:
ROIs are an electronic link with instruction on the tops and slots for comments on set drugs under review.
Carrier data call template:
The data was reformatted. The medical and pharmacy tabs were separated but are otherwise identical. Plan design added columns K-O to capture enrollee costs
Board questions: What parts of this form are legally required to be provided? Yellow fields are required; however the data must be presented without identifying the carrier.
The board considered a list of drugs and insulin products for affordability review according to the following criteria discussed at its February 19 meeting:
-
-
- Orphan drugs: Remove drugs with an orphan class designation.
- IRA CMS lists: Remove drugs that are on the IRA CMS negotiation list.
- Vaccines/HIV medications: Remove vaccines due to low patient cost and exclude HIV medications based on public comments and alignment with other state PDABs.
- Therapeutic alternatives: Prioritize drugs with two or more therapeutic alternatives to determine if market competition is impacting total annual spending and utilization.
- Generic drugs: Remove generics that have multiple therapeutic equivalents.
- Total spend: Focus on drugs with the highest annual net rebate spend per enrollee.
-
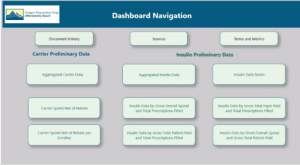
The board reviewed the dashboard.
The dashboard buttons link to underlying data: Carrier 2023 Preliminary aggregated information v03, Insulin 2023 Preliminary aggregated information based on APAC pharmacy data v01, Manufacturer 2023 annual increase v01, Manufacturer 2023 new specialty v01.
The board discussed which drugs should be included for the price limitation discussion later. There are 30 candidates, including insulin and non-insulin products. The goal is to choose a subset of drugs.
Non-insulin drug reviews
The board is reviewing drugs that don’t meet their criteria, and will request carrier data to address requirements to make selections based upon health inequities as identified in Section 2 of the legislation. That process reduced the list reduced to 34 drugs. Recommendations were made to remove drugs that are currently generic (removed Advair, Symbicort, Adderall XR, Farxiga, Flovent, Victoza….). Biktarvy was removed because it has a pediatric orphan drug designation.
27 drugs remain: Ajovy, Botox, Cosentyx, Creon, Dupixent, Eliquis, Emgality, Entresto, Humira, Ibrance, Jardiance, Mounjaro, Nurtec, Ocrevus, Odefsey, Ozempic, Perjeta, Rinvoq, Rybelsus, Taltz, Trelegy, Tremfya, Trulicity, Ubrelvy, Verzenio, Vraylar, and Xarelto.
The board voted to approve the prescription drug subset list above for affordability reviews as discussed by the board.
Insulin drugs
Next the board discussed the insulin drugs in the same manner. Levemir and Levemir FlexPen were flagged because they are no longer on the market.[i] Board members were concerned about their ability to understand biosimilars for insulins. No conclusions were drawn. The conversation will continue at the next meeting on April 16, 2025.
[i] It was discontinued on December 31, 2024. https://www.levemir.com/
The next PDAB meeting will be April 16, 2025 at 9 a.m. PST.
Standout resources and coverage
"PDAB chicanery: How drug affordability boards are undermining public engagement, Travis Manint, HIV/HCV Co-Infection Watch, March 30, 2025
"These boards, created under the guise of helping patients afford medications, are operating in ways that actively silence patient voices."