News
February 3, 2025: A New York jury convicts precursor sellers
Employees of the now-closed Chinese chemical company Hubei Amarvel Biotech were convicted at trial after shipping more than 200 kilograms of fentanyl precursor chemicals to the U.S. between November 2022 and June 2023.
[...]Partnership for Safe Medicines statement on weight loss drug ad blitz
Partnership for Safe Medicines released the following statement in response to the advertisement that lifestyle brand Hims & Hers released ahead of the Super Bowl to sell unregulated compounded weight loss drugs
[...]January 27, 2025: Another Canadian sentenced for fake prescription pill sales
Last week a Canadian man got 30 years for selling Americans counterfeit Xanax on the dark web and a New York spa owner was arrested after he allegedly injected patients with fake Botox
[...]January 21, 2025: PBMs pocketed billions by inflating medicine prices, says the FTC
A new FTC report examines how PBM business practices have inflated drug costs.
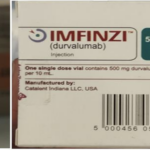
[...]WHO Alert: Counterfeit Cancer Medicines Reported in Europe
Information provided to WHO by AstraZeneca, the genuine manufacturer of IMFINZI, has confirmed that the products identified in this Alert are falsified. Laboratory analysis of samples of the falsified IMFINZI have been carried out by AstraZeneca. The analysis confirmed that the vials of the falsified product contained no active pharmaceutical ingredient.
[...]January 13, 2025: WHO warns about a fake cancer drug with English packaging
WHO reported a fake cancer treatment. This year’s Notorious Markets report focused on fake online pharmacies. Board member Andrea Thomas urged fraternities to stock Narcan.
[...]January 6, 2025: Counterfeit pills are increasingly available globally and their contents are ever more unpredictable
As prescription medications containing fentanyl and other illegal substances are becoming more widely accessible worldwide, a global crackdown on illicit drug precursors is leading cartels to experiment with the contents of their products.
[...]FDA Alert: Recall Issued on “Herbal” Diet Pill Containing Prescription and OTC Drug Ingredients
FDA analysis has found these products to contain undeclared Furosemide, Dexamethasone and Chlorpheniramine. Furosemide was found at 5.24 mg/g or 1.84 mg/capsule. Dexamethasone was found at 2.22 mg/g or 0.780 mg/capsule. Chlorpheniramine was found at 4.38 mg/g or 1.54 mg/capsule.
[...]December 16, 2024: Black market medicine in Texas and Tennessee
Two new cases involving black market medicine in Texas and Tennessee, and CPB seized thousands of pills in Laredo.
[...]December 9, 2024: Lawsuit against Snap to proceed
The lawsuit against Snap may proceed and the ADA issued a statement about compounded GLP-1 medicines.
[...]