News
Trump Administration Defies 20 Years of FDA Expertise and Guidance in Finalizing Politically Motivated Drug Importation Rule
Trump Administration Defies 20 Years of FDA Expertise and Guidance in Finalizing Politically Motivated Drug Importation Rule Washington, D.C. (September 24, 2020) – Shabbir Safdar, executive director of the Partnership for Safe Medicines, released the following statement in response to the Trump Administration’s Final Rule issued today on the importation of prescription drugs: “With 40…
[...]Final regulations released governing Canadian drug importation
On September 24th, 2020 the U.S. Department of Health and Human Services released a draft of finalized rulemaking for Importation of Prescription Drugs. Watch this space for the most up-to-date news.
[...]September 9, 2020 video: 1,000 Fake Pills per bottle – a bust in Toronto
Last June, police in Ontario, Canada carried out Project Javelin, shutting down a huge counterfeiting operation that manufactured hundreds of thousands of fentanyl pills disguised as Oxycocet, a Health Canada-approved generic oxycodone and acetaminophen product with the same ingredients as Percocet.
Update: Executive Director Shabbir Safdar followed up with retired Chief Superintendent of the Ontario Police Don Bell to talk about the impact these criminals could have had. Watch that interview in our September 23rd video, “Deadly Pills in Ontario.”
[...]Counterfeit Medicine News for the Week of September 14, 2020
In PSM’s round-up this week: 520,000 fake medical masks seized by Customs and Border Protection, another fake online pharmacy case, and a flood of news about counterfeit pills made with fentanyl.
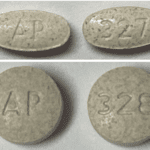
[...]FDA Alert: Acella Pharmaceuticals, LLC Issues Voluntary Nationwide Recall of Two Lots of NP Thyroid®, Thyroid Tablets, USP Due to Sub Potency
Risk Statement: Patients being treated for hypothyroidism (underactive thyroid), who receive sub potent NP Thyroid®, may experience signs and symptoms of hypothyroidism (underactive thyroid) which may include, fatigue, increased sensitivity to cold, constipation, dry skin, puffy face, hair loss, slow heart rate, depression, swelling of the thyroid gland and/or unexplained weight gain or difficulty losing weight. There is reasonable risk of serious injury in newborn infants or pregnant women with hypothyroidism including early miscarriage, fetal hyperthyroidism, and/or impairments to fetal neural and skeletal development. In elderly patients and patients with underlying cardiac disease toxic cardiac manifestations of hyperthyroidism may occur, such as cardiac pain, palpitations or cardiac arrhythmia. To date, Acella has received four reports of adverse events for these lot numbers possibly related to this recall.
[...]Counterfeit Medicine News for the Week of September 7, 2020
In PSM’s round-up this week: $132 million in losses from #covidscams since March, fake online pharmacies selling counterfeit and non-FDA approved opioids, Operation Quack Hack, and more.
[...]Counterfeit Medicine News for the Week of August 31, 2020
In PSM’s round-up this week: outrageous COVID-19-related financial fraud, fake treatments, and the week in deadly counterfeit pills.
[...]Preventing Rx Medications from becoming the next toilet paper during the Coronavirus Pandemic
If you are taking a life-saving HIV medication right now, or any kind of life saving medication, you may be concerned about how to be certain that your supply of medicine is secure. In this post, William Arnold of the Community Access National Network , PSM’s Shabbir Safdar, and Brandon M. Macsata of ADAP Advocacy Association offer suggestions to help.
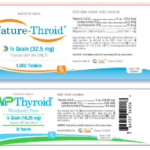
[...]RLC Labs, Inc., Issues Voluntary Nationwide Recall of All Lots of Nature-Throid® and WP Thyroid® with Current Expiry Due to Sub Potency
Risk Statement: Patients being treated for hypothyroidism (underactive thyroid), who receive sub potent Nature-Throid® or WP Thyroid®, may experience signs and symptoms of hypothyroidism (underactive thyroid) which may include, fatigue, increased sensitivity to cold, constipation, dry skin, puffy face, hair loss, slow heart rate, depression, swelling of the thyroid gland and/or unexplained weight gain or difficulty losing weight.
[...]CorgioMed LLC Issues Voluntary Nationwide Recall of All Lots of Leafree Instant Hand Sanitizer Aloe Vera Labeled as EDIBLE ALCOHOL
Risk Statement: Ingesting hand sanitizer, which is intended for topical use, may result in alcohol toxicity. Symptoms of alcohol toxicity may range from lack of coordination, slowed or slurred speech, drowsiness to coma, which can be fatal. Young children may experience a sharp decrease in blood sugar which may result in death. Pregnant women who ingest alcohol have experienced birth defects and developmental disabilities. Nursing mothers who ingest alcohol in above moderate levels may see developmental, growth and sleep pattern damages in their babies and may experience impaired judgement and ability to safely care for their child.
[...]