PSM News
PSM Statement on the passage of S.3201, which extends the DEA’s temporary scheduling of fentanyl-related substances as Schedule I for 15 months.
The Partnership for Safe Medicines (PSM) stands with our law enforcement partners in commending the U.S. Senate and the U.S. House of Representatives for their swift passage of S.3201, which would extend the DEA’s temporary scheduling of fentanyl-related substances to be Schedule I controlled substances for an additional 15 months. Without this reauthorization, criminals could…
[...]HHS Announces Dangerous Draft Regulations for Importing Drugs from Canada
Partnership for Safe Medicines Statement on Proposed Regulations to Import Prescription Medicines from Canada Washington, D.C. (December 18, 2019) – Shabbir Safdar, executive director of the Partnership for Safe Medicines, released the following statement in response to today’s announcement by the Trump Administration and the proposed regulations to allow importation of prescription medicines: “Citizens of…
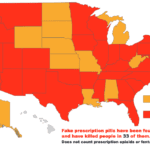
[...]PSM’s Executive Director Speaks on Morning Wakeup with Dave Akerly to Warn Michigan Residents about the Dangers of Drug Importation
PSM’s Executive Director Shabbir Safdar spoke with Dave Akerly on WILS in Lansing, Michigan about the importation proposal currently being debated in the Michigan House of Representatives. Safdar was enroute to the Michigan State House to participate in hearings on drug importation being held there. Here why our Executive Director has travelled to Michigan to testify at their drug importation hearing.
[...]Bloomberg Misses The Mark In Its Article About Drug Importation Advocacy
In case you missed it, Bloomberg’s Ben Elgin has published an “in-depth” piece on the connection between PhRMA and the Partnership for Safe Medicines. However, this has been public and widely reported on for years. More concerningly, the story misses the broader point about the clear safety concerns of foreign drug importation. Over the past…
[...]Partnership for Safe Medicines 2019 Congressional Briefings (September 24, 2019)
On Tuesday, September 24, 2019, counterfeit medication victims and their families, American and Canadian patient groups, local and federal law enforcement, and other experts in the fight against counterfeit medicines gathered in Washington, D.C. to discuss how importation proposals threaten to trigger drug shortages in Canada while wreaking havoc on medication safety for U.S. patients.
[...]Partnership for Safe Medicines’ Statement on Governor DeSantis Signing Florida’s Drug Importation Bill
Washington, D.C. (June 11, 2019) – Shabbir Imber Safdar, executive director of the Partnership for Safe Medicines, released the following statement today in response to the signing of Florida’s drug importation bill: “Today, Florida’s governor put politics over the safety of residents across the state. Although this was widely expected, we remain committed to protecting…
[...]Keynote Speaker Announced for Interchange
John Morton will be the keynote speaker for The Partnership for Safe Medicine’s Interchange on October 27, 2011 at the National Press Club.
Morton is the Director of Immigration and Customs Enforcement (ICE), the principal investigative arm of the U.S. Department of Homeland Security and the second largest investigative agency in the federal government. During his tenure at ICE, he has strengthened ICE’s investigative efforts, with a particular emphasis on border crimes, export controls, intellectual property enforcement and child exploitation.
Fake Drug Market Tripled Since 2004 – PSM board member quoted
USA Today has reported on the growing problem of fake drugs in the world markets. Counterfeit drugs made in Asia and other emerging markets are a growing problem that’s endangering consumers’ health. Since 2004, the number of fake drug incidents has tripled to 1,700, says the Pharmaceutical Security Institute (PSI), which estimates the size of…
[...]PSM Vice President Expecting Full House for Counterfeit Drug Conference
Each year, Partnership for Safe Medicines Vice President Dr. Bryan Liang hosts a health policy conference in San Diego. We’re happy to report that all seats have been filled for his March 26, 2010 conference, “Pharmaceutical Crime: Investigating and Prosecuting Drug Diversion and Counterfeiting,” sponsored by the Institute of Health Law Studies at the California…
[...]A Second Article
Take a look at the Safe Medicines Blog.
[...]