About PSM
Statement on Trump Administration Executive Order on Drug Importation
Washington, D.C. (July 24, 2020) – Shabbir Safdar, executive director of the Partnership for Safe Medicines, released the following statement in response to President Trump’s executive order signed today on the importation of prescription drugs:
[...]PSM Statement on the New Pilot Program from NTIA and FDA
The Partnership for Safe Medicines (PSM) applauds the Food and Drug Administration (FDA) and the National Telecommunications and Information Administration (NTIA) pilot program established this week to curb unapproved opioids illegally available online by potentially suspending or blocking offending domain names.
[...]Criminal Internet Pharmacy Networks Are Capitalizing on COVID-19.
In May 2020, the National Association of Boards of Pharmacy (NABP) released Rogue Online Pharmacies in the Time of Pandemic: Capitalizing on Misinformation and Fear, which focuses on how established fake pharmacy networks have pivoted to cash in on the coronavirus. PSM’s illustrated version of the report offers a compact summary of NABP’s work. Watch…
[...]Download our “5 Types of #covidscams” bookmark
Our #covidscams bookmark educates patients about five kinds of online crime that have spiked since the pandemic. Download a copy for yourself and visit our covid-19 scams tracking page to catch up on the news.
[...]Policy Resolution on Counterfeit Device Destruction, May 8, 2020
The following policy resolution was passed unanimously at the May 8, 2020 Advisory Board meeting: The Partnership for Safe Medicines believes that counterfeit medical devices are dangerous to American patients and that the U.S. Food and Drug Administration (FDA) should have the unequivocal power to seize and destroy these dangerous products whether they are found…
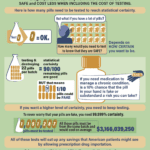
[...]How Many Tests Need to Be Performed to Know That a Batch of Pills is Safe?
Testing medicine for legitimacy is a complicated process. Across 24 different prescription medicines, the average cost to test a single dose is $2,750. However, ensuring that a batch of 100 pills is 90% certain to be safe requires testing at least 22 pills. Achieving 99.999% certainty requires even more testing, at tremendous expense. To learn more about this topic, read PSM’s summary: safedr.ug/Acri-Explained.
[...]Avoid COVID scams by buying medicine safely – Read our tips in English and en Español
Download our guide, AVOID SCAMS & COUNTERFEITS: Quick Tips to Safely Purchase Medicines Online (in English | en Español) to learn more about how to protect yourself and your loved ones from fake COVID-19 treatments.
[...]HHS comments come in overwhelmingly against Canadian drug importation proposal
Yesterday ended a 78-day comment period for the White House’s proposal to import medicine from Canada. In all, over 1,000 comments were filed. Overwhelmingly, these comments opposed the proposed rule or expressed skepticism that the rule could meet the two requirements listed in the Medicare Modernization Act of 2003: be safe and save consumers money. In fact, when you read the comments, it is clear that this policy is overwhelming opposed by experts on the issues of economics and medicine safety.
[...]Former FDA Associate Commissioner Warns of the “Massive Safety Risks of Importation”
Source: Twitter This editorial by Peter J. Pitts was published in The Times Weekly on March 3, 2020. Mr. Pitts is president of the Center for Medicine in the Public Interest and a former FDA associate commissioner. Keep Canadian drugs out of U.S. medicine cabinets The Trump administration recently proposed two rules that would allow…
[...]