Press Releases
PSM submits comment on CBP’s proposed rules to improve screening of low-value shipments
On March 14, the Partnership for Safe Medicines submitted comment on Customs and Border Protection’s proposed update to regulations around low-value, “de minimus” shipments.
[...]New report reveals illegal ingredients for knockoff weight loss drugs flooding into U.S. from foreign sources, endangering patient safety
The Partnership for Safe Medicines today released a new report that found suspicious, unauthorized, and illegal ingredients for popular diabetes and obesity injectables (commonly known as weight loss drugs) are flooding into the U.S. from foreign sources despite U.S. laws forbidding them from coming through the border.
[...]Partnership for Safe Medicines statement on weight loss drug ad blitz
Partnership for Safe Medicines released the following statement in response to the advertisement that lifestyle brand Hims & Hers released ahead of the Super Bowl to sell unregulated compounded weight loss drugs
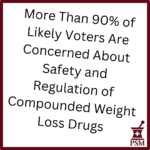
[...]PSM Compounded Weight Loss Drugs Poll
PSM commissioned a survey about compounded diabetes and weight loss injectable drugs. 1,000 likely voters across political affiliations were crystal clear — they are worried about safety and the current lack of oversight. See the full results.
[...]PSM applauds the reintroduction of the SHOP SAFE Act
On June 13, 2024, PSM sent a letter to Congressmen Darrell Issa and Jerrold Nadler applauding the reintroduction of the Stopping Harmful Offers on Platforms by Screening Against Fake E-Commerce (SHOP SAFE) Act.
[...]The Partnership for Safe Medicines supports pharmacy benefit reforms in California Senate Bill 966
State Senator Scott Wiener’s bill would eliminate under-reimbursement practices that create a dangerous opportunity for criminals to enter the legitimate supply chain.
[...]PSM applauds NNOAC statement of concern about Canadian drug importation
“We are deeply concerned about the impact importation of unapproved foreign prescription drugs will have on counterfeit medication in the United States, especially as FDA considers approving additional SIPs.”
[...]The Partnership for Safe Medicines supports S. 2943, the SHOP SAFE Act
On January 17, 2024 PSM wrote the Senate Subcommittee on Intellectual Property to endorse S.2934, which would expand the Trademark Act of 1946 to extend liability for selling harmful counterfeits to online platforms. Read the letter here.
[...]Partnership for Safe Medicines Condemns the Weakening of the U.S. Drug Supply through Canadian Drug Importation + Full coverage of decision
PSM’s statement on the FDA decision and full coverage including media stories, official documents, and statements from stakeholders.
[...]PSM Executive Director to Speak at USPTO Roundtable on Counterfeits
Shabbir Imber Safdar, executive director of the Partnership for Safe Medicines, will participate in the upcoming United States Patent and Trademark Office Roundtable: Future strategies in anti-counterfeiting and anti-piracy.
[...]