Press Releases
Dr. Marv Shepherd, founding member of PSM, announces retirement from the board
The Board of Directors for the Partnership for Safe Medicines (PSM) announces the retirement of Board President Dr. Marvin D. Shepherd.
[...]Partnership for Safe Medicines Statement on Pres. Biden’s Remarks on Lowering Prescription Drug Costs
Washington, D.C. (August 12, 2021) – Shabbir Safdar, executive director of the Partnership for Safe Medicines, released the following statement in response to President Biden’s remarks about lowering healthcare costs for Americans
[...]Partnership for Safe Medicines Statement on the Centers for Disease Control and Prevention’s Drug Death Totals for 2020
Shabbir Safdar, executive director of the Partnership for Safe Medicines (PSM), released this statement in response to the Centers for Disease Control and Prevention’s (CDC) preliminary statistics for the number of Americans whose deaths in 2020 involved drugs
[...]Partnership for Safe Medicines Statement on Biden Administration Executive Order to Foster Competition
Shabbir Safdar, executive director of the Partnership for Safe Medicines, released this statement in response to President Biden’s executive order to foster competition, which favors importation of prescription drugs from Canada.
[...]Illegal Pill Presses Pose Serious, Nationwide Threat to American Patients and Communities
The Partnership for Safe Medicines and the National Association of Drug Diversion Investigators have released an update to 2019’s Illegal Pill Presses: An Overlooked Threat to American Patients.
Since the initial report, fentanyl deaths are higher than ever and these pills – created by clandestine pill presses around the globe – continue to be sold on the streets and on the dark web.
[...]Partnership for Safe Medicines’ Statement on Congressional Action to Permanently Schedule Fentanyl-Related Analogues and Substances
Shabbir Safdar, Executive Director of the Partnership for Safe Medicines, released the following statement today in response to Congressional actions to permanently scheduling illicitly manufactured and deadly fentanyl…
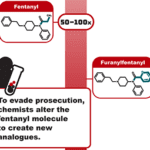
[...]What is “Permanently Scheduling” Fentanyl-related Substances and Analogues, and Why is it so Important?
Learn why permanently scheduling fentanyl-related substances and analogues on Schedule I of the Controlled Substances Act is an important policy tool in the fight against fentanyl-laced counterfeit medicines.
[...]PSM Applauds Senate Passage of the Safeguarding Therapeutics Act
The Partnership for Safe Medicines (PSM) applauds the passage of H.R. 5663 – the Safeguarding Therapeutics Act. The bipartisan bill, introduced by Congressman Brett Guthrie (KY-02-R) and Congressman Eliot Engel (NY-16-D), was passed via unanimous consent in the Senate on December 8th.
[...]Statement on Litigation Challenging Legality of Administration’s Final Rule Permitting State-Sponsored Drug Importation From Canada
Today, PhRMA, The Partnership for Safe Medicines and Council for Affordable Health Coverage initiated litigation in the U.S. District Court for the District of Columbia challenging action by HHS and FDA permitting pharmacists and wholesalers, pursuant to state-sponsored programs, to import certain prescription drugs from Canada into the United States without drug manufacturers’ authorization or oversight.
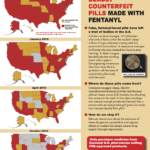
[...]Deadly Counterfeit Pills Found in All 50 U.S. States; Deaths Now Reported in 42 of Them.
With the recent report that police in Maui, Hawaii seized 400 counterfeit oxycodone pills made with fentanyl on October 2, 2020, the United States has reached a sobering milestone: public sources have reported about fake pills made with fentanyl in all 50 states.
[...]