Alert
FDA Class 1 Recall: Imported, Non-FDA Approved COVID-19 Tests
Use of these tests may cause serious adverse health consequences or death. There is also a risk of injury if users follow any labeling instructions directing self-collection of nasopharyngeal or oropharyngeal samples.
[...]FDA Alert: Dietary Supplement Recalled Due to Prescription Drug Ingredients
Proper Trade LLC/My Stellar Lifestyle was notified by Amazon that laboratory analysis has found the product to be tainted with tadalafil, an ingredient in FDA approved products for treatment of male erectile dysfunction in the family of drugs known as phosphodiesterase (PDE-5) inhibitors.
[...]FDA Alert: Class 1 Recall of Unauthorized COVID-19 Tests
North American Diagnostics is recalling these tests because they were distributed to U.S. customers without authorization, clearance, or approval from the FDA. North American Diagnostics did not provide the FDA with adequate validation data to show that the test’s performance is accurate. Use of these tests may cause serious adverse health consequences or death.
[...]FDA Alert: Dietary Supplement Sold on Amazon Recalled for Presence of Prescription Drug Ingredients
The product was distributed via the internet and fulfilled by Amazon nationwide in the USA. On December 17, 2020, FDA issued a press release that warned consumers to avoid certain products found on Amazon, eBay and other retailers due to hidden and potentially dangerous drug ingredients. It also encouraged online marketplaces to ensure these products are not sold on their platforms.
[...]FDA Alert: Dietary Supplement Marketed for Pain Relief Recalled Due to Presence of Diclofenac and Dexamethasone
Consumption of undeclared diclofenac could result in serious adverse events that include cardiovascular, gastrointestinal, renal, and anaphylaxis in patients taking concomitant NSAIDs and/or anticoagulants, in those who have allergies to diclofenac, or those with underlying illnesses. Consumption of undeclared dexamethasone may lead to severe and serious adverse events such as adrenal suppression, a disorder in which the adrenal glands do not produce enough hormones, and adverse consequences can range from limited adverse consequences to death.
[...]FDA Alert: Class 1 Recall of Some Mesa Biotech COVID Tests Due to Risk of False Positives/Contamination
This is a reprint of an FDA Alert. Mesa Biotech, Inc., Recalls Certain Accula SARS-CoV-2 Tests for Risk of False Positives Caused by Contamination The FDA has identified this as a Class I recall, the most serious type of recall. Use of these devices may cause serious injuries or death. Recalled Product Product Name: Accula…
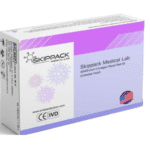
[...]FDA Alert: Class 1 Recall on Skippack Medical Lab SARS-CoV-2 Antigen Rapid Test
This is a reprint of an FDA Alert. Do Not Use Skippack Medical Lab SARS-CoV-2 Antigen Rapid Test: FDA Safety Communication May 10, 2022 The U.S. Food and Drug Administration (FDA) is warning people not to use the Skippack Medical Lab SARS-CoV-2 Antigen Rapid Test (Colloidal Gold). This test is not authorized, cleared, or approved…
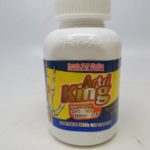
[...]FDA Alert: Herbal Arthritis Treatments Contain Dangerous Prescription Drugs
FDA has received adverse event reports, including of liver toxicity and death, associated with the use of Artri King products, since the agency issued its first warning about an Artri Ajo King product on January 5, 2022.
[...]FDA Alert: Class 1 Recall of Unauthorized Covid-19 Tests
This is a reprint of an FDA Alert. SD Biosensor Recalls STANDARD Q COVID-19 Ag Home Tests That Are Not Authorized, Cleared, or Approved by the FDA and May Give False Results The FDA has identified this as a Class I recall, the most serious type of recall. Use of these devices may cause serious…
[...]FDA Issues Warning About Unauthorized Covid-19 At-Home Tests
The U.S. Food and Drug Administration (FDA) is warning people not to use the SD Biosensor Inc. STANDARD Q COVID-19 Ag Home Test. The test is not authorized, cleared, or approved by the FDA for distribution or use in the United States. The FDA is concerned about the risk of false results when using this unauthorized test. This unauthorized test may be packaged in a white and magenta box.
[...]