Alert
FDA Issues Warning About Unauthorized Covid-19 At-Home Tests
The U.S. Food and Drug Administration (FDA) is warning people not to use the SD Biosensor Inc. STANDARD Q COVID-19 Ag Home Test. The test is not authorized, cleared, or approved by the FDA for distribution or use in the United States. The FDA is concerned about the risk of false results when using this unauthorized test. This unauthorized test may be packaged in a white and magenta box.
[...]Texas eBay Seller Recalls Diet Supplements Containing Banned Sibutramine
Am eBay seller is voluntarily recalling all lots of Hydro Pineapple Burn to consumer level because FDA analysis has found the product to contain undeclared sibutramine, an appetite suppressant that was withdrawn from the market because of safety issues.
[...]California Boutique Recalls Diet Supplements For Containing Banned Sibutramine
Je Dois L’avoir Boutique is voluntarily recalling all of the 365 Skinny High Intensity Pills and or 365 Skinny Emergency Boutique, 30 day capsules supply to the retail/consumer level. The 365 Skinny High Intensity Pills and 365 Skinny Emergency Boutique have been found to contain Sibutramine which is a controlled substance by the DEA.
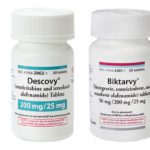
[...]Counterfeit HIV Drug Alert: Gilead Warns of Fake Versions of Biktarvy and Descovy Sold to U.S. Pharmacies
Gilead has alerted potentially impacted pharmacies to investigate the potential for counterfeit or tampered Gilead medication sold by distributors not authorized by Gilead that may be within their recent supply and to remain vigilant to the potential for this to occur in the future. The authenticity and safety of Gilead-branded medicines can only be secure when obtained directly through Gilead’s authorized distributors.
[...]FDA Alert: Third Dietary Supplement Sold on Amazon Recalled for Containing Prescription Drug Ingredients
On December 17, 2020, FDA issued a press release that warned consumers to avoid certain products found on Amazon, eBay and other retailers due to hidden and potentially dangerous drug ingredients. It also encouraged online marketplaces to ensure these products are not sold on their platforms.
[...]FDA Alert: Second Dietary Supplement Recalled for Containing Prescription Drug Ingredients
On December 17, 2020, FDA issued a press release that warned consumers to avoid certain products found on Amazon, eBay and other retailers due to hidden and potentially dangerous drug ingredients. It also encouraged online marketplaces to ensure these products are not sold on their platforms.
[...]FDA Alert: Dietary Supplement Recalled for Containing Prescription Drug Ingredients
Consumers should contact their physician or healthcare provider if they have experienced any problems that may be related to using these drug products.
[...]ED-Marketed Dietary Supplement Recalled Due to Presence of Prescription Drugs
This recall has been initiated after an FDA laboratory analysis found the product to contain undeclared sildenafil and/or tadalafil. Sildenafil and tadalafil are ingredients in FDA approved products for the treatment of male erectile dysfunction in the family of drugs known as phosphodiesterase (PDE-5) inhibitors. The presence of sildenafil and/or tadalafil in Adam’s Secret Extra Strength products makes them unapproved new drugs for which the safety and efficacy have not been established and therefore subject to recall.
[...]FDA Puts All Mexico-Produced Hand Sanitizers on Import Alert
Under the import alert, alcohol-based hand sanitizers from Mexico offered for import are subject to heightened FDA scrutiny, and FDA staff may detain the shipment. As part of their entry review, FDA staff will consider any specific evidence offered by importers or manufacturers that the hand sanitizers were manufactured according to U.S. current good manufacturing practice requirements. This marks the first time the FDA has issued a countrywide import alert for any category of drug product.
[...]Methanol-Contaminated Rubbing Alcohol Recalled by FDA
This is a reprint of an FDA Alert. Essaar Inc. Issues Voluntary Nationwide Recall of Rubbing Alcohol Contaminated with Methanol When a company announces a recall, market withdrawal, or safety alert, the FDA posts the company’s announcement as a public service. FDA does not endorse either the product or the company. Company Name: Essaar Inc.…
[...]