Importation
National Sheriffs’ Association Sends Letter of Concern About Importation of Prescription Drugs
On August 8, 2018, the National Sheriffs’ Association wrote the President to oppose prescription drug importation. Importing drugs from other countries, they say, “jeopardizes law enforcement’s ability to protect the public health and endangers the safety of law enforcement and other first responders.”
[...]Drug Importation is Fraught with Peril
As a licensed pharmacist, I’m all too familiar with patients’ difficulties getting medications they need and their physician has prescribed. As baby boomers age, pharmacists see more patients at our counters unable to obtain needed treatments for heart disease, high blood pressure, diabetes, and other chronic illnesses. This issue is now being acknowledged and a healthy debate has begun over possible solutions. But one idea policymakers shouldn’t pursue is opening up our country’s secure drug supply to medicines coming from outside our borders.
[...]Talk Radio Host Reminds Americans that Drug Reimportation Poses Real Risks
In this August 9, 2018 editorial for RealClearMarkets, Bill Martinez reminds Americans that the FDA hasn’t yet approved importation for a reason: it’s impossible to open the floodgates to foreign drug imports without cutting corners on safety.
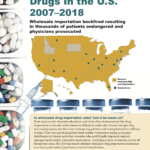
[...]Between 2007 and 2018 Foreign Wholesalers Sold American Doctors Unreliable Black Market Cancer Drugs
From 2007 to 2018 American physicians and clinics demonstrated that drug importation is not safe and is extremely difficult to make safe. Doctors thought they were saving money. Instead they purchased illegally imported, expired, damaged and outright counterfeit medications—including cancer treatments—from black market wholesalers posing as licensed distributors in Canada and other countries.
[...]Partnership for Safe Medicines Statement on FDA Working Group on Drug Importation
Washington (July 19, 2018) – Shabbir Imber Safdar, executive director of the Partnership for Safe Medicines, released the following statement regarding today’s announcement by the Department of Health and Human Services to create a drug importation working group at FDA: “We are deeply concerned about today’s announcement, particularly given the deaths of Americans in at…
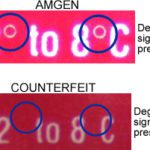
[...]Drug Importation, Counterfeit Medications and the Pharmacist’s Liability: A Case Study and Legal Precedent
For the last 15 years, the FDA and HHS have opposed drug importation for safety reasons, but there is another question that is often overlooked: If a pharmacy inadvertently distributes a counterfeit drug it legally purchased from a foreign wholesaler, can the pharmacist be held liable? A 2004 lawsuit, Fagan v. AmerisourceBergen Co, raises disquieting questions.
[...]Medicine Importation Legislation is a Smuggler’s Dream
While the Trump administration works to stem the raging epidemic of opioid addiction, Sen. Bernie Sanders, I-Vt., has a plan to make it worse. He calls it the Affordable and Safe Prescription Drug Importation Act, S. 469. This legislation would open our borders to the free flow of drugs – all drugs – from Canada and other countries.
It would be more accurate to call it the Unsafe Opioid Importation Facilitation Act – and we can’t afford it. Sanders is still peddling the bogus line that importing prescription drugs from Canada is the ticket to lower health-care bills for Americans. Not only has Bernie’s tonic been exposed as a fraud, it is downright deadly. Read more…
[...]Former FBI staffer argues that we cannot deter drug counterfeiters without harsher penalties
In a June 7, 2018 editorial for The Hill, Former FBI deputy assistant director and Pharmaceutical Security Institute President Thomas Kubic argues that current sentences and penalties are not strong enough to deter criminal drug importation: “It boils down to this, if an entity — be it an organization or person — opts to engage in activities involving illegal distribution chains, there should be consequences, often certain, swift, and overwhelmingly punitive.”
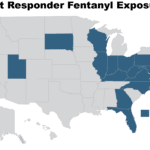
[...]Mothers of Counterfeit Drug Victims Join Law Enforcement, Pharmacy Experts to Raise Alarm Over Lethal Fentanyl Imports
Partnership for Safe Medicines Announces Creation of Fentanyl Council to Spotlight Impact on U.S. Law Enforcement, Explore Proliferation of Pill Presses
[...]Partnership For Safe Medicines Urges Passage of the STOP Act to Increase Inspections for Dangerous Synthetic Opioids
Partnership For Safe Medicines Urges Passage of the STOP Act to Increase Inspections for Dangerous Synthetic Opioids Legislation will increase surveillance of primary smuggling route for fentanyl WASHINGTON (May 24, 2018) – The Partnership for Safe Medicines (PSM) today urged passage of the Synthetics Trafficking and Overdose Prevention Act of 2017 (S.372), also known as…
[...]