Importation
Drug Importation Proposal Increases Opportunity for Fentanyl to Cross Border, Experts Say
University of Maryland professor Robert Freeman warns that proposed changes in U.S. laws and regulations to make the movement of drugs from other countries easier will increase fentanyl deaths in the U.S. In an editorial in The Hill, Dr. Freeman warns that the opioid abuse problem affects a greater proportion of the Canadian population…
[...]The American Pharmacists Association Writes Congress to Oppose Importation
On March 22, the American Pharmacists Association (APhA), which represents 64,000 pharmacists, pharmacy technicians, pharmacy students and other pharmacy professionals wrote a letter to Congress opposing plans to legalize importation of non-FDA approved drugs. These proposals, they wrote, are “in direct conflict with recent efforts by Congress and federal agencies to increase the integrity and security of the U.S. drug supply.”
[...]Former FDA Commissioners Warn About Drug Importation
The Washington Post reports that the four most recent FDA commissioners have warned Congress that American patients will suffer if they import drugs from other countries, citing fake, substandard and contaminated drugs as potential consequences.
[...]The Partnership for Safe Medicines Congressional Briefing March 16 -17, 2017
In 2017, the danger of counterfeit drugs is serious and ubiquitous in America, touching all 50 states. Large-scale smugglers bring in fake cancer drugs containing nothing but mold and water and sell them at a discount to oncologists. Fake pills containing deadly doses of fentanyl appear in the supply chain either from fake online pharmacies…
[...]Pew Trust Warns Senator Sanders that Drug Import Bill Could Compromise the Safety of the U.S. Drug Supply
On Tuesday, February 28, Allan Coukell, Senior Director of Health Programs for the Pew Charitable Trusts, wrote Senator Bernie Sanders to ask that he not undermine safety protections for medication with the Affordable and Safe Prescription Drug Importation Act.
[...]Inconvenient facts about drug importation proponents don’t want you to hear
Canadian pharmacists are not allowed to dispense drugs to Americans with just a prescription from an American physician. The National Association of Pharmacy Regulatory Authorities (NAPRA), which represents pharmacy regulators across Canada, says that “pharmacists in Canada are not legally allowed to fill prescriptions from physicians that are not licensed to practice medicine in Canada.”…
[...]The Partnership for Safe Medicines Letter to Congress – February 28, 2017
The Partnership for Safe Medicines (PSM) today sent a letter to all members of Congress urging them to continue protecting Americans and stand against efforts that would allow substandard and even dangerous counterfeit medicines to flow freely across U.S. borders and into the hands of the American people. The letter, signed by nearly 170 groups, is…
[...]NABP Warns Congress About Canadian Online Pharmacies, February 10, 2017
A clandestine fake drug factory in Colombia washes empty medication bottles before filling them with counterfeit Tazocin, a prescription antibiotic. Photo courtesy of Pfizer. On February 10th, 2017, the National Association of Boards of Pharmacies wrote a letter to the U.S. Congress about the risks associated with Canadian online pharmacies. The letter, below in text…
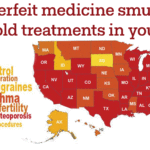
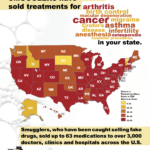
[...]Bipartisan Safety Issues: How Many Different Kinds of Black Market Medicines have been Sold in Your State?
Since 2012, the FDA has issued warnings to more than 3,000 doctors, clinics and hospitals about eight different breaches in the U.S. drug supply chain by rogue distributors such as Richards Pharma, Canada Drugs, Medical Device King, Gallant Pharmaceuticals, and TC Medical. These rogue distributors offered 63 different non-FDA approved medicines. Have you or has someone you know taken medicine that these smugglers have sold?
[...]Cosmetic Injectable Treatments Now Used to Treat Dozens of Ailments so Fakes Poses A Danger to More U. S. Patients
In 2016 alone, over 1,400 practitioners were warned by the FDA that their supplier was selling unapproved so-called “Botox.” With more than 2,400 professionals warned in the past 5 years, the expansion of use for this medication provides new markets for counterfeiters selling fake and misbranded drugs. Botulinum neurotoxin, or Botox as it is known,…
[...]