Importation
Counterfeit Medicine News for the Week of July 27, 2020
In PSM’s round-up this week: Continuing COVID-19 fraud, counterfeit Botox, and ongoing counterfeit pill news.
[...]July 29, 2020 video: Importation Executive Order Explainer
On July 24, 2020, the White House issued an executive order to implement three approaches to foreign drug importation, all of which involve dipping into other countries’ drug supplies and putting them in U.S. medicine cabinets. Watch this week’s video to learn more.
[...]Citizens Against Government Waste Warns Drug Importation Executive Orders Will Cost Patients in More Ways Than One
In this July 28, 2020 editorial published in the WasteWatcher blog, Elizabeth Wright argues that the administration’s Executive Order will “encourage more illegal behavior and a greater production of counterfeit drugs from countries like China, Mexico, and India.” Wright is the Director of Health and Public Policy for Citizens Against Government Waste
[...]Counterfeit Medicine News for the Week of July 20, 2020
In PSM’s round-up this week: PSM’s statement about drug importation, The Safeguarding Therapeutics Act, continuing COVID-19 fraud, and ongoing counterfeit pill news.
[...]Counterfeit Medicine News for the Week of July 13, 2020
PSM’s round-up this week includes fake PPE and COVID-19 treatments, deaths and hospitalizations related to hand sanitizer made of methanol, fentanyl pill seizures, and guilty pleas from two Ukrainian men who sold counterfeit cancer and hepatitis treatments to U.S. agents.
[...]May 20, 2020 video: Maine Importation Update
Maine submitted a state importation plan to the U.S. Department of Health and Human Services (HHS) recently to meet its own May 1, 2020 deadline. Given the deep structural flaws in both the idea of importing medicine from Canada and in Maine’s application to do so, PSM wonders if there is a better use of Maine taxpayer dollars than to continue to pursue this idea.
[...]Research Study Confirms That Cheap And Safe Drug Importation Is Not Feasible
Research Study Confirms That Cheap And Safe Drug Importation Is Not Feasible Read the abstract for Dr. Acri’s article. In March 2020, the Journal of Pharmaceutical Health Services Research, an official journal of the Royal Pharmaceutical Society, published a study by Dr. Kristina M.L. Acri née Lybecker titled “State Pharmaceutical Importation Programmes: An Analysis of…
[...]Freedom Of Access Act Response From The State of Maine
In June 2019, the governor of Maine signed into law LD 1272, a bill that would allow the state to establish a wholesale Canadian prescription drug importation program. On April 13, 2020, the Partnership for Safe Medicines (PSM) filed a request to receive copies of all submitted responses to Maine’s Request for Comment (RFC) for…
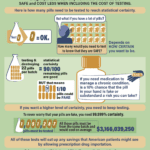
[...]How Many Tests Need to Be Performed to Know That a Batch of Pills is Safe?
Testing medicine for legitimacy is a complicated process. Across 24 different prescription medicines, the average cost to test a single dose is $2,750. However, ensuring that a batch of 100 pills is 90% certain to be safe requires testing at least 22 pills. Achieving 99.999% certainty requires even more testing, at tremendous expense. To learn more about this topic, read PSM’s summary: safedr.ug/Acri-Explained.
[...]Eight Surprising Things We Learned from Reading the 1,200 Comments Filed with the FDA on Canadian Drug Importation
For 78 days, the Department of Health and Human Services accepted public comment on the proposed rulemaking that would allow states to establish drug importation programs, individuals and organizations submitted 1,210 comments, and PSM read each of them. Here are what we feel are the most important takeaways…
[...]