Article Topic
Med spas need stronger regulation
Medical spas and wellness clinics are operating on the edge of existing regulatory frameworks and it’s a risk to American patients.
[...]Prescription Drug Affordability Board Activity, August 2025
Prescription Drug Affordability Board Activity, August 2025 Activities Summary Colorado: Colorado’s PDAB met on August 22, 2025 for to continue rulemaking to set an upper payment limit for Enbrel, during which they heard substantial public comment. They will continue this process at their next meeting on October 3. Maryland: The next PDAB meeting will be…
[...]September 15, 2025: Telehealth company must stop implying its compounded GLP-1 is FDA-approved, warns FDA
The warning letter was one of about 100 is a new focus on deceptive direct-to-consumer drug advertisements.
[...]September 8, 2025: FDA launches an import alert listing safe GLP-1 manufacturers
The “green list” will help protect patients from unreliable, illegally compounded GLP-1s and simplify screening for border security.
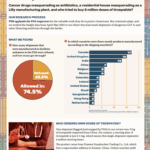
[...]PSM leads letter in support of an FDA mandate to destroy high-risk imports
PSM led a coalition of organizations urging leaders of the Senate HELP and House Energy and Commerce committees to help strengthen the FDA’s ability to protect Americans from unsafe and counterfeit medicines and medical products.
[...]10 years of fentanyl pills
A decade ago no one had heard of deadly counterfeit prescription pills. Victims’ families bravely spoke up, lobbied, and rallied for change.
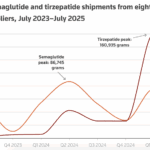
[...]September 2, 2025: U.S. compounding restrictions have stemmed a flood of compounded semaglutide and tirzepatide
Chinese companies that supplied U.S. compounders have turned to making generic semaglutide for markets where Novo Nordisk’s main patent is expiring in 2026.
[...]Affidavits, USA v Rebecca Fadanelli, October 2024
United States District Court District of Massachusetts USA v Rebecca Fadanelli Affidavits Filed October 2024 Read the documents: 1 | 2
[...]Plea Agreement, USA v Facial Expressions, June 2025
United States District Court Eastern District of Kentucky Southern Division, London USA v Facial Expressions Plea Agreement Filed June 10, 2025 Read the document.
[...]Information, USA v Facial Expressions June 2025
United States District Court Eastern District of Kentucky Southern Division, London USA v Facial Expressions Information Filed June 9, 2025 Read the document.
[...]