Article Topic
#covidscams episode for April 15, 2020
In this edition: ASOP letter to the White House, more fake test kits and treatments and HR 5663, the Safeguarding Therapeutics Act
[...]ASOP and 40 Patient Organizations Reach Out to Vice President Pence to Put a Stop to #Covidscams
In a letter dated April 9th, the Alliance for Safe Online Pharmacies (ASOP) has requested that the Vice President prioritize evidence-based messaging and focus energies on provable therapies for treating COVID-19. The letter also asks the Vice President to address the “systemic, structural Internet policy problems that enable COVID-19 scams online.”
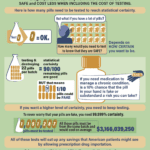
[...]How Many Tests Need to Be Performed to Know That a Batch of Pills is Safe?
Testing medicine for legitimacy is a complicated process. Across 24 different prescription medicines, the average cost to test a single dose is $2,750. However, ensuring that a batch of 100 pills is 90% certain to be safe requires testing at least 22 pills. Achieving 99.999% certainty requires even more testing, at tremendous expense. To learn more about this topic, read PSM’s summary: safedr.ug/Acri-Explained.
[...]#covidscams episode for April 8, 2020: LegitScript report, fake cures, fake testing sites, and covidscam bingo!
In this edition: LegitScript’s comprehensive report on COVID-19 cyberscams, U.K. man accused of selling fake COVID-19 cures, unauthorized COVID-19 testing sites in Kentucky,
[...]COVID-19 Scams Video Update, March 30, 2020
In this edition: Rise of Fake ‘Corona Cures’ Revealed in Global Counterfeit Medicine Operation, Fake ‘COVID-19 Testing Kits’ Across North America, FDA Letter: Do Not Use Chloroquine Phosphate Intended for Fish as Treatment for COVID-19, and L.A. Warns of Coronavirus Consumer Issues.
[...]Counterfeit Surgical Masks Seized During Operation Pangea, Fake COVID-19 Test Kits Seized by CBP
Operation Pangea found more than 34,000 bogus surgical masks among the 4.4 million illicit pharmaceuticals and 37,000 counterfeit medical devices seized during their seven-day global effort to target counterfeit drug crime. In Los Angeles, U.S. Customs and Border Protection seized counterfeit coronavirus test kits. #covidscams are on the rise.
[...]FDA Alert: Do Not Use Chloroquine Phosphate Intended for Fish as Treatment for COVID-19 in Humans
The FDA’s Center for Veterinary Medicine has recently become aware that some consumers may mistake chloroquine phosphate used to treat disease in aquarium fish for FDA-approved drugs (used to treat malaria and certain other conditions in humans) that are being studied as a COVID-19 treatment for humans. Unfortunately, we have learned that one person in the United States has died after he and his wife reportedly took chloroquine used to treat their fish in an attempt to prevent COVID-19; his wife also became very ill. We are continuing to investigate this incident.
[...]Life-threatening counterfeit drugs taught Rick Roberts that medicine safety can’t be taken for granted
Life-threatening counterfeit drugs taught Rick Roberts that medicine safety can’t be taken for granted. Safe medicines advocate Rick Roberts opening the Senate briefing, September 24, 2019. Rick Roberts, a professor at the University of San Francisco and a member of The Partnership for Safe Medicines’ Advisory Board, began thinking about the problem of medicine safety…
[...]Eight Surprising Things We Learned from Reading the 1,200 Comments Filed with the FDA on Canadian Drug Importation
For 78 days, the Department of Health and Human Services accepted public comment on the proposed rulemaking that would allow states to establish drug importation programs, individuals and organizations submitted 1,210 comments, and PSM read each of them. Here are what we feel are the most important takeaways…
[...]Executive Director of Colon Cancer Association Warns Against “Ill-Considered Drug Importation Scheme”
This editorial by Andrew Spiegel was published in The International Business Times on March 23, 2020. Mr. Spiegel is executive director of the Global Colon Cancer Association and Chair of the World Patient Alliance. President Trump, Price Controls Can’t Combat Coronavirus The U.S. outbreak of novel coronavirus, COVID-19, has quickly evolved into a national nightmare.…
[...]