Article Topic
FDA Alert: Med Man Expands Voluntary Nationwide Recall of Up2 and Bow & Arrow Due to Presence of Undeclared Sildenafil
This is a reprint of an FDA Alert. When a company announces a recall, market withdrawal, or safety alert, the FDA posts the company’s announcement as a public service. FDA does not endorse either the product or the company. Company Announcement Date: February 24, 2020 FDA Publish Date: February 24, 2020 Product Type: Dietary Supplements…
[...]FDA Worked With Indian Government To Seize 500 Counterfeit Drug Shipments in January
The U.S. Food and Drug Administration (FDA) has just announced a successful joint operation with the Government of India targeting counterfeit prescription drugs, counterfeit over-the-counter medications, fake medical devices, and misbranded dietary supplements containing harmful ingredients.
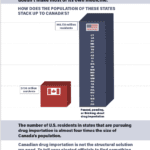
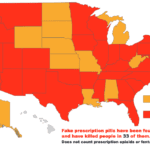
[...]Canadian Importation Does Not Work
The number of U.S. residents in states that are considering importing drugs from Canada is almost four times Canada’s own population. Canadian drug importation is not the structural solution we need.
[...]Resources for organizers fighting dangerous Canadian drug importation
Resources for organizers on the HHS Canadian drug importation comment period Ways you can help spread the word Submit your own comments or tell people to go to http://safedr.ug/takeaction to submit their own. Email your community and ask them to comment. Follow us on Facebook and share our calls-to-action with your community. Resources Read PSM’s…
[...]PSM files comments on HHS’s proposed Canadian drug importation rules; cites danger to patients
Today The Partnership for Safe Medicines filed comments with Health and Human Services about the dangers posed by its draft regulations for state-based Canadian drug importation programs. PSM cited historic problems with and patient harm from Canadian vendors selling counterfeit medications to U.S. patients and medical practices; showed broad opposition to the plan by Canadian stakeholders; and provides alternatives that don’t impact patient safety.
[...]Counterfeit Medicines Are a Problem in the European Union—A Problem that is Continuing to Grow
According to a report from Europol’s Intellectual Property Crime Threat Assessment 2019, “Counterfeit pharmaceuticals pose a growing threat to the EU, affecting a large number of Member States. A wide and increasingly diverse range of medicines is targeted by counterfeiters.”
The Europol ICP Threat Assessment reports that there has been an increase in seizures of counterfeit drugs used in the treatment of serious illnesses, as well as a growing number of counterfeit drug incidents affecting the legal drug supply chain.
Security Breach Illustrates Another Danger of Fake Online Pharmacies
Planet Drugs Direct, an online pharmacy based in Winnipeg, Canada, has announced a data breach. Hackers broke into their servers, exposing customers’ names, medical details, and contact and banking information. Legitscript is unequivocal in calling Planet Drugs Direct a “Rogue Internet Pharmacy,” their worst rating for online pharmacies, accusing them of violating state and federal laws.
[...]Infographic: Counterfeiting By The Numbers
Our infographic, “Counterfeiting by the Numbers,” highlights facts from the U.S. Department of Homeland Security’s January 2020 report, Combating Trafficking in Counterfeit and Pirated Goods, which documents the extraordinary scale of the global counterfeiting market and its effects across all economic sectors—including medicines.
[...]PSM Statement on the passage of S.3201, which extends the DEA’s temporary scheduling of fentanyl-related substances as Schedule I for 15 months.
The Partnership for Safe Medicines (PSM) stands with our law enforcement partners in commending the U.S. Senate and the U.S. House of Representatives for their swift passage of S.3201, which would extend the DEA’s temporary scheduling of fentanyl-related substances to be Schedule I controlled substances for an additional 15 months. Without this reauthorization, criminals could…
[...]New Jersey Enacts Increased Penalties for Counterfeit Drug Crimes
The Governor of New Jersey has just signed A-5037, a new law designed to increase penalties for counterfeit drug crimes committed in the state.
[...]