Posts Tagged ‘FDA Alert’
FDA Alert: Update on Previous Nationwide Warning About Presence of Undeclared API in Capsules
The FDA published an update to a previous alert about undeclared active pharmaceutical ingredients in a dietary supplement to expand the package styles affected by this warning.
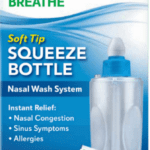
[...]FDA Alert: Recall Issued on Nasal Wash System Due to Microbial Contamination
An FDA alert shared information on a nationwide recall of a nasal wash system due to microbrial contamination.
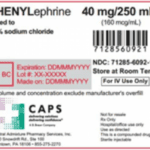
[...]FDA Alert: Recall Issued on Injectable Product Due to Potential Particulate Matter
An FDA alert warned that Central Admixture Pharmacy Services issued a nationwide recall of Phenylephrine 40 mg added to 0.9% Sodium Chloride 250 mL in 250 mL Excel Bag due to the detection of black particulate matter in a single sealed vial of Phenylephrine Hydrochloride.
[...]FDA Alert: Recall Issued on Dietary Supplement Containing Active Pharmaceutical Ingredient
An FDA alert warned that Vitafer-L has been found to contain undeclared tadalafil, an active pharmaceutical ingredient. Products containing tadalafil cannot be marketed as dietary supplements.
[...]FDA Alert: Dietary Supplement Recalled for Containing Prescription Drug Ingredients
This is a reprint of an FDA Alert.
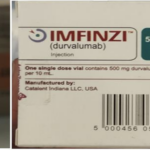
[...]WHO Alert: Counterfeit Cancer Medicines Reported in Europe
Information provided to WHO by AstraZeneca, the genuine manufacturer of IMFINZI, has confirmed that the products identified in this Alert are falsified. Laboratory analysis of samples of the falsified IMFINZI have been carried out by AstraZeneca. The analysis confirmed that the vials of the falsified product contained no active pharmaceutical ingredient.
[...]FDA Alert: Recall Issued on “Herbal” Diet Pill Containing Prescription and OTC Drug Ingredients
FDA analysis has found these products to contain undeclared Furosemide, Dexamethasone and Chlorpheniramine. Furosemide was found at 5.24 mg/g or 1.84 mg/capsule. Dexamethasone was found at 2.22 mg/g or 0.780 mg/capsule. Chlorpheniramine was found at 4.38 mg/g or 1.54 mg/capsule.
[...]FDA Alert: Dietary Supplement Recalled Due to Presence of Sildenafil and Diclofenac
Consumption of products with undeclared sildenafil may interact with nitrates found in some prescription drugs (such as nitroglycerin) and may cause a significant drop in blood pressure that may be life threatening. People with diabetes, high blood pressure, high cholesterol, or heart disease often take nitrates. Among the adult male population, who are most likely to use this product, adult males who use nitrates for cardiac conditions are most at risk.
[...]FDA Alert: Dietary Supplement Contains Drug That Can Cause Heart Attack, Stroke and Internal Bleeding
Use of products with undeclared diclofenac may cause increased risk of cardiovascular events, such as heart attack and stroke, as well as serious gastrointestinal damage, including bleeding, ulceration, and fatal perforation of the stomach and intestines. This hidden drug ingredient may also interact with other medications and significantly increase the risk of adverse events, particularly when consumers use multiple NSAID-containing products.
[...]FDA Alert: Compounding Pharmacy in California Warned After Particulates Found in Injections
On August 14, 2024, FDA received a complaint from a patient who noticed a black particulate in a vial of semaglutide distributed by Fullerton Wellness. On September 23, 2024, FDA received information from California regulatory authorities as part of ongoing collaboration between FDA and the state noting deficiencies found at Fullerton Wellness during a state inspection. After the state inspection, Fullerton Wellness voluntarily ceased operations.
[...]