Posts Tagged ‘FDA Alert’
FDA Alert: FDA Reminds Compounders to Use Appropriate and Sterile Ingredients
The agency urges manufacturers, including repackagers, to clearly identify any ingredients intended for use in foods or dietary supplements on the label. Providing this information on ingredient labels could help prevent compounders from using ingredients that are not appropriate for sterile drugs and may help prevent patient harm.
[...]FDA Alert: Pain Relief-Marketed Dietary Supplement Recalled For Containing Prescription Steroids, Muscle Relaxants, and OTC Pain Relievers
Consumers taking this product should immediately consult with their health care professional to safely discontinue use of this product. The risks of withdrawal from corticosteroids should be assessed by a healthcare professional. Only licensed health care professionals can evaluate patients for the risk, or confirm the existence, of adrenal suppression. Consumers that have product which is being recalled should return to place of purchase or discard.
[...]FDA Alert: Herbal Supplement Recalled for Containing Prescription Steroids and OTC Antihistamines
If used chronically at the recommended dose, dexamethasone could cause severe and serious adverse events such as adrenal suppression (a disorder in which the adrenal glands do not produce enough hormones), central nervous system and psychiatric/behavioral effects, weight gain, gastrointestinal effects, elevated blood glucose, increased infection risks, neuromuscular and skeletal side effects, ocular effects, cardiovascular effects, dermatologic effects endocrine and metabolic issues, among other adverse events not mentioned.
[...]FDA Alert: Dietary supplement sold on Amazon recalled for containing prescription drug ingredients
This is a reprint of an FDA Alert. Boulla LLC Issues Voluntary Nationwide Recall of Boom Max Capsules Due to the Potential Presence of Undeclared Sildenafil When a company announces a recall, market withdrawal, or safety alert, the FDA posts the company’s announcement as a public service. FDA does not endorse either the product or…
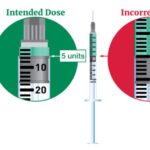
[...]FDA alerts health care providers, compounders and patients of dosing errors associated with compounded injectable semaglutide products
FDA has received reports of adverse events, some requiring hospitalization, that may be related to overdoses due to dosing errors associated with compounded semaglutide injectable products.
[...]FDA Alert: Florida Company’s Dietary Supplement Recalled for Containing Prescription Drug Ingredients
STOP CLOPEZ CORP is voluntarily recalling one lot of Schwinnng capsules to the consumer level. FDA analysis has found the Schwinnng products to contain Nortadalafil. Nortadalafil is an active drug ingredient known for the treatment of male erectile dysfunction. The presence of Nortadalafil in Schwinnng capsules makes it an unapproved new drug for which the safety and efficacy have not been established and, therefore subject to recall.
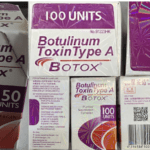
[...]FDA alert: Counterfeit version of Botox found in multiple states
The products appear to have been purchased from unlicensed sources. Medications purchased from unlicensed sources may be misbranded, adulterated, counterfeit, contaminated, improperly stored and transported, ineffective and/or unsafe.
[...]FDA warns consumers not to use counterfeit Ozempic (semaglutide) found in U.S. drug supply chain
FDA continues to investigate counterfeit Ozempic (semaglutide) injection 1 milligram (mg) in the legitimate U.S. drug supply chain and has seized thousands of units of the product. FDA is aware of five adverse events from this lot.
[...]FDA Alert: Ginseng Compound Recalled for Containing Undeclared Drug Ingredients
This is a reprint of an FDA Alert. 8th Avenue Pharmacy Issues Voluntary Nationwide Recall of Notoginseng Formula Special Gout Granule Due to the Presence of Hidden Drug Ingredients, Diclofenac and Dexamethasone When a company announces a recall, market withdrawal, or safety alert, the FDA posts the company’s announcement as a public service. FDA does…
[...]FDA Alert: Pain Relief Dietary Supplements Recalled Due to Presence of Diclofenac
Risk Statement: Consumption of undeclared diclofenac could result in serious adverse events that include cardiovascular, gastrointestinal, renal, and anaphylaxis in patients taking concomitant NSAIDs and/or anticoagulants, such as Warfarin, in those who have allergies to diclofenac, or those with underlying cardiovascular, gastrointestinal, renal, and hepatic illnesses.
[...]