Posts Tagged ‘importation’
Prescription Drug Freight Fraud Report, November 2025
Our analysis of data in September and October 2025 revealed that large-scale freight fraud involving GLP-1s, oncology drugs, and other high-demand pharmaceuticals continues unabated.
[...]Prescription Drug Freight Fraud Report, October 2025
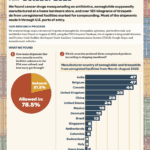
What large-scale commercial imports made it into the country between March and August of 2025?
[...]Prescription Drug Freight Fraud Report, September 2025
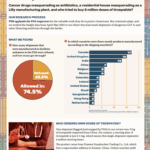
Our analysis of data from June and July revealed hundreds of pharmaceutical shipments that entered the U.S. from facilities no one would expect, including unregistered Chinese exporters and alternative medicine clinics abroad.
[...]Prescription Drug Freight Fraud Report, July 2025
What if your tirzepatide shipment came from a Brazilian beauty clinic? Or a vial of semaglutide was manufactured, supposedly, at a Costco in Toronto? In April and May 2025, dozens of shipments of semaglutide, tirzepatide, apixaban, and antibiotics entered the U.S. from facilities that aren’t in the FDA’s drug manufacturing database. These aren’t low-volume personal-use…
[...]Prescription Drug Freight Fraud Report, May 2025
What if your blood thinners were made in an unverified location in Colombia? In March 2025, dozens of pharmaceutical shipments entered the U.S. that were manufactured in places no one would expect.
[...]FOIA Returns from towns using Alternative Funding Vendors
PSM has sent open records requests to several towns and schools with self-funded health plans about their relationships with Alternative Funding Vendors.
[...]Handout: Illegal ingredients linked to knockoff weight loss drugs pouring in from foreign sources
Compounded versions of GLP-1 injectable treatments for diabetes and obesity have surged in popularity despite a lack the safety and efficacy assurances. The FDA has warned that these knockoff versions sometimes contain illicit semaglutide or tirzepatide—the active pharmaceutical ingredients (APIs) in weight loss drugs. Working with George Karavetsos, former director of FDA’s Office of Criminal…
[...]Why won’t Canadian drug importation work?
Why won’t Canadian drug importation work? Download our factsheet: Why won’t Canadian importation work?
[...]Pandemic counterfeits demonstrate the danger of Canadian drug importation
Pandemic counterfeits demonstrate the danger of Canadian drug importation Download our factsheet Pandemic counterfeits demonstrate the danger of Canadian drug importation.
[...]What Canadians are saying about importation
What Canadians are saying about importation Download our factsheet citing Canadian opinions about importation..
[...]