News Coverage
The Partnership for Safe Medicines has been publishing information about the counterfeit drug problem around the world for more than a decade. With experts leading the organization and a committed and passionate set of writers and editors, our content is more in-depth than many other sources, which simply copy links to the news from other websites.
Mike Leavitt | March 20, 2017 With the issue of prescription drug importation being debated on Capitol Hill again, mark me in the skeptical camp. As a matter of safety and practical policymaking, drug importation simply doesn’t work. It is not by happenstance that our country has the world’s safest drug supply. Counterfeit medicines are proliferating…
On Tuesday, February 28, Allan Coukell, Senior Director of Health Programs for the Pew Charitable Trusts, wrote Senator Bernie Sanders to ask that he not undermine safety protections for medication with the Affordable and Safe Prescription Drug Importation Act.
Investigators confirm that pills found near Chloe Kotval’s body were fake Percocet containing a lethal dose of fentanyl. She is the youngest Canadian to be killed by fentanyl-laced fake medication, but hardly the first. Chloe Kotval, a 14 year old Ottawa high school student died on February 14, 2017 after taking a counterfeit pill laced…
Americans have been talking about saving money on medicine by importing drugs from Canada for years and circulating price comparison tables to prove their point. In the past few days, this graphic has been making the rounds; The Partnership for Safe Medicines staff wondered whether these prices were accurate, and so we checked them by…
For years, if you suffered from severe allergies, EpiPen was the best-known option to prevent anaphylaxis. Mylan, the manufacturer of EpiPen, released a generic alternative in December 2016, however, there is an even more affordable option on the market. As reported by Consumer Reports, Adrenaclick had long been a cheaper alternative to the EpiPen, but…
Coalition Calls on Congress to Maintain Safe Access to Medicines For Immediate Release February 28, 2017 Washington, D.C. – The Partnership for Safe Medicines (PSM) today sent a letter signed by nearly 170 groups to all members of Congress urging them to continue protecting Americans and stand against efforts that would allow substandard and even dangerous…
David Beckford was one of 5 California residents charged in a scheme to produce and sell fake Xanax from bulk medication imported from China. A Northern California Federal Court has sentenced Oakland resident David Beckford to ten years in prison after his guilty plea on counterfeit drug, money laundering, and weapons charges, The Department of…
NYC Couple Pleads Guilty to Conspiring to Illegally Distribute and Dispense Fake Cosmetic Injectable
Brooklyn, New York residents Bu Young Kim and Chan Hui Cho pleaded guilty in federal court to conspiring to dispense and administer cosmetic drugs and treatment without being a licensed medical professional, import of misbranded drugs and attempting to smuggle $79,986 from the United States to South Korea. According to the United States Department of…
A clandestine fake drug factory in Colombia washes empty medication bottles before filling them with counterfeit Tazocin, a prescription antibiotic. Photo courtesy of Pfizer. On February 10th, 2017, the National Association of Boards of Pharmacies wrote a letter to the U.S. Congress about the risks associated with Canadian online pharmacies. The letter, below in text…
Juan Gallinal, a former Virginia police officer, acted as the head of a Florida conspiracy that used a valid brick-and-mortar pharmacy as a cover for series of fake pharmacy websites that sold misbranded and addictive drugs. The owner of a fake online pharmacy business in Florida has been sentenced to eight years in prison, the…
The Justice Department, the United States Food and Drug Administration (FDA) and the Drug Enforcement Administration (DEA) are concerned about Americans illegally purchasing prescription medicines from foreign sources. According to News Channel 10, investigations are underway to protect Americans from these potentially dangerous substances. The Justice Department has been looking at the data and as…
Arizona resident Betty Hunter died of lung cancer, but how much longer would she have lived if her oncologist had not treated her with counterfeit Avastin? A recent Danish documentary tells the tragic story of Arizona grandmother Betty Hunter, who in 2011 sought treatment for her lung cancer at an oncology clinic in Chandler Arizona.…
An investigation into the corruption of former Governor Javier Duarte has uncovered allegations that children with cancer were treated in state-run hospitals in Veracruz with counterfeit chemotherapy medicines due to budget shortfalls and lack of resources caused by his administration. The BBC reports, that when tested, the counterfeit chemotherapy drugs came back as an inert…
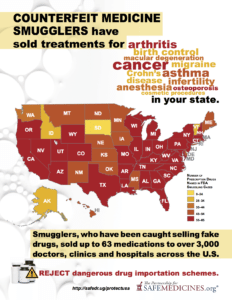
Since 2012, the FDA has issued warnings to more than 3,000 doctors, clinics and hospitals about eight different breaches in the U.S. drug supply chain by rogue distributors such as Richards Pharma, Canada Drugs, Medical Device King, Gallant Pharmaceuticals, and TC Medical. These rogue distributors offered 63 different non-FDA approved medicines. Have you or has someone you know taken medicine that these smugglers have sold?
The two men acted as U.S. distributors for a series of Thailand-based fake online pharmacy websites that sold imported and non-FDA approved medication to U.S. consumers. Troy Tapia and Anthony Rouse III of Louisiana have each been sentenced to three years probation after pleading guilty to fraud conspiracy, wire fraud, mail fraud and money laundering,…
In 2016 alone, over 1,400 practitioners were warned by the FDA that their supplier was selling unapproved so-called “Botox.” With more than 2,400 professionals warned in the past 5 years, the expansion of use for this medication provides new markets for counterfeiters selling fake and misbranded drugs. Botulinum neurotoxin, or Botox as it is known,…
January 5, 2017 Dear Senators and Representatives, In December of 2015 we released the attached letter warning Congress of the dangers to American patients from counterfeit drugs brought into America through “importation” proposals. As you consider the budget this year, we wanted to bring to your attention several recent tragic events that show the negative…
Defendants allegedly purchased expired or close-to-expired gastric lap-band surgery kits on the Internet, disguised them as new, and then re-sold them to local physicians. Florida residents Peter Kakfa and Gregory Grimm are being accused of running an expired/misbranded gastric bypass banding system scam via their employer Apollo Endosurgery, the Department of Justice (DOJ) reports. According…
Authorities in San Juan are making a public appeal to find more victims of “Rosa from Venezuela.” A federal court in San Juan, Puerto Rico has brought charges of injecting adulterated products into at least 2 different women against Rosa Betancourt-Farfán, the Food and Drug Administration (FDA) reports. Betancourt-Farfán is alleged to have posed as…
Pair that were arrested in London in October 2012 for operating a fake online pharmacy that sold to U.S. consumers plead guilty and are sentenced. Pakistani fake online pharmacy entrepreneurs Sheikh Waseem Ul Haq and Tahir Saeed have been sentenced to time served after pleading guilty to 48 counts, including “conspiracy to import Schedule II,…
Dr. Magdy Elsawy has admitted to no wrongdoing in his settlement, however it was originally alleged he provided non-FDA approved cancer medications to his patients, defrauded Medicare, and provided treatments to patients that were more complex than needed or entirely medically unneeded. North Iowa Today is reporting that the owner and lead physician at Hematology…
Dr. Diana Anda Norbergs was convicted by a Federal Grand Jury on 45 counts related to her multi-year practice of treating her oncology patients with imported, non-FDA approved cancer medications, and then billing Medicare, public, and private insurers for the full cost of the genuine treatments. The U.S. Department of Justice (DOJ) is reporting that…
California nurse who provided Botox treatments to the stars of Real Housewives of Orange County has pleaded guilty to charges she treated her patients with non-FDA approved Botox. Her original supplier of the misbranded Botox was SB Medical, an illicit Canadian medication importer that paid $75 million in fines and Penalties in 2015 for illegally…
Indicted in absentia in 2012, Junaid Qadir, part owner of a clandestine medication importer based in Pakistan has finally been forced to face U.S. justice. Junaid Qadir, a principal in a family-owned pharmaceutical business in Karachi, Pakistan, has been sentenced to 2 years in prison for his role in the illegal importation and sale of…
Rooting out fake online pharmacy links from University websites is an endless game of whack-a-mole for University network security officers. Ephemeral pharmacy web links appear and disappear constantly on major university websites. Inspired by one of their news gathering partners, Fox 59 News in Indianapolis, Indiana did a bit of checking with the help of…
With the costs of medication rising, consumers are on the lookout for discounts on the drugs they need. PSM’s Timothy Mackey offered CNN some helpful tips on how to ensure the online pharmacy you are doing business with is a legitimate source of medication. From generic drugs to prescription drug coupons and prescription assistance programs,…
The United Kingdom’s Medicines & Healthcare Regulatory Agency (MHRA) has launched an information and safety campaign concerning dangerous fake diet pills. The MHRA is asking U.K. residents to not take chances with lose-weight-fast promises with their “Dodgy Diet Pills” campaign, warning consumers that “fake or unlicensed medical products sold as slimming pills are untested,” and…
Dr. Paul S. Singh is currently serving one year of home detention after pleading guilty to charges that he deceived his patients and defrauded Medicare by implanting his patients with non-FDA approved IUDs purchased on the Internet. Earlier this year, a Southern California gynecologist, Dr. Paul S. Singh was sentenced to 6 months in prison,…
South Carolina medical practice prosecuted for buying non-FDA approved injectable cosmetic treatments. A medical practice in Greenville, South Carolina has been sentenced to 3 years of federal probation for treating patients with non-FDA approved Botox, a Department of Justice (DOJ) press release reported on the occasion of the sentencing. According to the DOJ, “Records obtained…
As the price of Mylan EpiPens have skyrocketed, Consumer Reports and the National Association of Boards of Pharmacies caution consumers to save money safely by taking advantage of available discounts, and steering clear of fake online pharmacies. Consumer Reports has reached out to consumers in light of recent cost hikes for life-saving EpiPens. According to…
The director of The Food and Drug Administration’s Office of Criminal Investigations (FDA-OCI) warns consumers about counterfeit medication. George Karavetsos, the director of FDA-OCI, discussed patient risks from counterfeit medication in a recent episode of the daytime TV show, “The Doctors,” and shared a short film called FDA Supplement Truth that illustrates the dangers posed…
When Prince Rogers Nelson died in April, it was reported that he died of an apparent overdose of opiates, which he had used to combat chronic hip pain. Now a new report from Minneapolis indicates he died as a result of taking counterfeit hydrocodone containing deadly fentanyl. Prince Rogers Nelson, an American musician and performer,…
Karen Chamberlain faces up to a year in prison on charges she purchased cancer drugs from clandestine medical importer, Quality Specialty Products (QSP). A 62-year old medical office manager in Tennessee is facing up to a year in prison after pleading guilty to misdemeanor charges that she imported non-FDA approved cancer medications for the clinic…
The National Association of Boards of Pharmacy also examines the role that fake online pharmacies are playing in distributing counterfeit pain pills laced with fentanyl. A new report from the National Association of Boards of Pharmacy (NABP) has just been released. Titled “The Internet Drug Outlet Identification Program Progress Report for State and Federal Regulators:…
As counterfeit pills containing various fentanyl analogues are killing people all over the United States, the DEA takes a measure of this deadly and growing counterfeit drug epidemic. The recent arrival of large amounts of “counterfeit prescription drugs containing fentanyls” is causing an explosive growth in overdose deaths in the United States, according to a…
In an article written by Next Avenue’s Emily Gurnon, Partnership for Safe Medicines board member Jim Dahl explains why shopping at unlicensed online pharmacies is anything but harmless to U.S. consumers. While patients might be lured to unlicensed pharmacy website with the promise of savings, a counterfeit drug that doesn’t help your health is no…
Although 5 doctors in Connecticut and 256 doctors nationwide have received FDA warning letters concerning purchases of non-FDA approved medication from the now-defunct medicine wholesaler Gallant Pharma International, no state medical boards have investigated any of their practices. Now Senator Richard Blumenthal of Connecticut is calling for the FDA to provide these names to state…
This is a reprint of an FDA Alert. When a company announces a recall, market withdrawal, or safety alert, the FDA posts the company’s announcement as a public service. FDA does not endorse either the product or the company. For Immediate Release July 1, 2016 Contact Consumers Dream Body Weight Loss websales@dreambodyweightloss.com (888)882-7612 Grand Prairie,…
Update: On December 13, 2017, the U.S. Court of Appeals for the Second Circuit vacated William Scully’s conviction because a lower court “declined to allow Scully to introduce evidence at his 2015 trial showing he sought legal advice about importing drugs with foreign labels from one of his lawyers.” Ultimately Scully pleaded guilty to one charge of…
In its 9th year, the International Week of Action brought together enforcement organizations from 115 countries throughout the globe in an effort of eliminate the sales of counterfeit medication online. The 9th Interpol-led effort Operation Pangea just completed its efforts, according to a June 9th press release from the Food and Drug Administration (FDA). George…
Dr. Mimlitz specialized in treating men who complained of a lack of energy. His treatments were actually unapproved drugs from South Korea purchased from Mexico via Internet sources. A Saint Louis medical doctor, Dr. Michael Mimlitz, has pleaded guilty to charges of introducing misbranded drugs into Interstate commerce, according to his waiver of indictment. According…
This is a reprint of an FDA Alert. Company Announcement When a company announces a recall, market withdrawal, or safety alert, the FDA posts the company’s announcement as a public service. FDA does not endorse either the product or the company. For Immediate Release: June 7, 2016 Contact Consumers: thebodyshotbarinfo@yahoo.com, (910)-849-3348 Media: Tiffany Braswell (910)-849-3348 The Body Shot Bar is…
Creator of the “Mineral Miracle Solution” sentenced to prison for peddling a compound not fit for human consumption. Some of his followers still advocate its use, however. In 2015, the Department of Justice (DOJ) reported that Louis Daniel Smith had been sentenced to 4 years and 3 months in prison for creating and marketing a…
Bust by Ramsey County Sheriff’s Department is the second time the same Saint Paul southeast Asian market has been raided. In 2013, seven vendors were charged for selling non-FDA approved medication. A recent raid at a Hmong night market in Minnesota has turned up pills, needles, syringes and expired medication, according to a report in…
Venezuelan man posed as a doctor at a Florida esthetician clinic, and injected victim with non-FDA approved substance, which triggered her death. The case is similar to the rash of cases that have recently been uncovered in Texas. Jose Robusto, a Venezuelan sought in the aesthetic treatment death of 28 year old Suyima Torres, has…
Website visitors now see a seizure banner displaying in place of the fake drug advertisements The US Department of Justice (DOJ) has announced that 67 different domain names for commercial websites “engaged in the illegal sale and distribution of counterfeit and prescription drugs” have had seizure orders executed against them by U.S. Immigration and Customs…
Cancer doctor working in Lodi, California and his wife, who acted as his office administrator admit no wrongdoing, but pay Federal Government $300,000 to settle allegations that they treated patients with counterfeit, imported cancer medication. The U.S. Department of Justice (DOJ) announced May 9th that Dr. John F. Kiraly and wife Rena Kiraly have agreed…
Counterfeiting of drugs has exploded since we last had a serious debate about the importation of branded drugs. In just one year, 2013, the Pharmaceutical Security Institute reports that worldwide incidents of pharmaceutical crime rose almost 9%. During one week in 2013, the FDA, in partnership with Interpol, seized $41 million worth of illegal or counterfeit medicines, and shut down over 1,600 illegal online pharmacies. Mexico is a major global source of those fake drugs. Its illicit trade stands at an estimated $650 million per year–equal to 10% of its total drug sales.
NBC news show examines the clandestine business of administering non-FDA approved beauty treatments. In the new episode of American Greed, titled “Vanity and Greed: Deadly Beauty”, the NBC show looks at the story of two convicted practitioners of counterfeit beauty treatments. Mississippi resident Tracey Lynn Garner was found guilty of murder in the death of…
Dr. Ann Kinnealey can no longer practice medicine in Illinois after a hearing by the Illinois State Medical Board determined that she purchased misbranded cancer medication from foreign supplier, Quality Specialty Products (QSP). QSP was a wholesale subsidiary of Canadian Internet Pharmacy giant, CanadaDrugs. Dr. Kinnealey had her 2 medical licenses suspended on April 21…
Though their medication it sold was neither FDA-approved nor legally imported, Gallant Pharma was able to pass itself off as a legitimate pharmaceutical supplier simply by basing their operation in a Washington D. C. suburb, and claiming their drugs were sourced from Canada. When the Department of Justice (DOJ) originally announced the indictment of 11…
This is a reprint of an FDA Alert. [Posted 04/01/2016] AUDIENCE: Surgery, OB/GYN, Risk Manager ISSUE: The FDA is aware of allegations that Boston Scientific’s urogynecologic surgical mesh may contain counterfeit raw material. We are examining these allegations to determine any necessary and appropriate next steps. FDA is not currently aware that the alleged counterfeit…
Darlene Krueger and her co-conspirators sold Chinese-made, “all-natural” weight loss supplements via website and mail order sales. Operating out of Louisiana and Florida, Krueger repackaged and sold bulk illicit medications containing the banned weight-loss drug sibutramine, which she then passed off as herbal supplements to her unsuspecting customers. On March 23, 2016, The Food and…
A 37-year-old mother is dead after taking what she thought was the anti-anxiety medication Xanax. Her family is asking authorities to investigate her death. In January 2016, Misty Burnett took pills that she thought were Xanax, reports News Channel 5. Shortly thereafter Misty’s 18-year-old daughter discovered her mother dead. Alicia Allred, the girl’s aunt told…
A 19-year-old boy and a 29-year-old man in Santa Cruz are both dead after ingesting a fatal dose of fentanyl hidden in fake Xanax pills. A 22-year-old man is facing charges that he supplied the fatal fakes. The San Francisco Chronicle reported that on October 24th, 2015, two young men at a party took what…
Late in 2015, a group of San Francisco friends took what they thought was the anti-anxiety medication, Xanax. Within 48 hours, three of them were dead due to overdoses of the powerful end-stage cancer painkiller, fentanyl. Three residents of San Francisco have died as a result of taking counterfeit Xanax, according to KRON4 local news…
Nicholas Brandt-Sorenson, a professional cyclist who was suspended from racing for 2 years after testing positive for banned substances, has now pleaded guilty to misdemeanor charges he illegally imported Chinese-made drugs for resale through his website. The Los Angeles Times reports that disgraced professional cyclist Nicholas Brandt-Sorenson, has admitted that he purchased foreign-produced performance-enhancing drugs,…
The 2012 fake Avastin warnings were just the tip of the iceberg for what is now unfolding into one of the most convoluted counterfeit medication incidents that has ever been uncovered. The supply of fake cancer medication has been traced to Internet pharmacy giant CanadaDrugs and in the last year, 4 doctors have been prosecuted…
Texas authorities at both the regional and Federal level have combatting a rash of injuries and deaths caused by counterfeit cosmetic injections. From fake dermal fillers and other counterfeit versions of beauty treatments to industrial silicone injections sealed with superglue, these fake injectable cosmetic peddlers are putting the lives of Texas women at risk. The Dallas…
In 2014, Federal Prosecutors indicted 3 Texans on charges that they were importing counterfeit versions of prescription medications from China for resale in the United States. This month, the last of the 3 has been sentenced to 15 months in prison. On February 3, 2016, the Department of Justice (DOJ) announced that Catherine Nix had…
After a 3-year effort to bring him to justice, Junaid Qadir has been indicted in Federal court on charges he illegally imported bulk counterfeit medications into the United States. The United States Department of Justice (DOJ) has announced that a Karachi native has been extradited from Germany to face counterfeit drug charges in the United…
Robert Lohr sold the fakes via a fake pharmacy known as either “American Drug Club of Bradenton” or “Canadian American Drug Club.” Lohr made over $1 million selling his counterfeit medication to unsuspecting patients. The United States Department of Justice (DOJ) reports that Bradenton, Florida resident Robert Lohr has pleaded guilty to conspiracy to smuggle…
This is a reprint of an FDA Alert. Recall: Firm Press Release FDA posts press releases and other notices of recalls and market withdrawals from the firms involved as a service to consumers, the media, and other interested parties. FDA does not endorse either the product or the company. For Immediate Release January 28, 2016…
A County officials warn public about the toxic threat that fake medications pose, and share a tragic story about a woman whose efforts to save money by using non-FDA approved treatments led to her death. An NBC News story shares the details of a counterfeit medication death in Los Angeles County. Esmeralda Mendez described for…
This is a reprint of an FDA Alert. FDA posts press releases and other notices of recalls and market withdrawals from the firms involved as a service to consumers, the media, and other interested parties. FDA does not endorse either the product or the company. Recall: Firm Press Release For Immediate Release January 20, 2016…
This is a reprint of an FDA Alert. Recall: Firm Press Release FDA posts press releases and other notices of recalls and market withdrawals from the firms involved as a service to consumers, the media, and other interested parties. FDA does not endorse either the product or the company. For Immediate Release December 22, 2015…
This is a reprint of an FDA Alert. Recall: Firm Press Release FDA posts press releases and other notices of recalls and market withdrawals from the firms involved as a service to consumers, the media, and other interested parties. FDA does not endorse either the product or the company. For Immediate Release December 23, 2015…
A new campaign by International Institute for Research Against Counterfeit Medicines (IRACM) aims to educate patients that anyone with access to the Internet can be exposed to counterfeit drugs via fake online pharmacies. Did you realize that of 331,430 websites monitored by Legitscript, a whopping 94.3% are selling pharmaceuticals illegally? The Wild West nature of…
This is a reprint of an FDA Alert. Recall: Firm Press Release FDA posts press releases and other notices of recalls and market withdrawals from the firms involved as a service to consumers, the media, and other interested parties. FDA does not endorse either the product or the company. For Immediate Release December 9, 2015…
This is a reprint of an FDA Alert. FDA posts press releases and other notices of recalls and market withdrawals from the firms involved as a service to consumers, the media, and other interested parties. FDA does not endorse either the product or the company. Recall: Firm Press Release For Immediate Release …
Mississippi Attorney General Jim Hood, who has made significant contributions to state-based efforts to protect Americans from counterfeit products, offered an attorney general’s perspective on working collaboratively with Federal agencies to fight counterfeit medicines.
This is a reprint of an FDA Alert. FDA posts press releases and other notices of recalls and market withdrawals from the firms involved as a service to consumers, the media, and other interested parties. FDA does not endorse either the product or the company. December 3, 2015 Contact Consumers Julio Tapia (718) 415-2611…
Dr. Shepherd was interviewed on a local Austin radio station and described for listeners the realities of the counterfeit medication economy, and the dangers U.S. citizens face from imported medication. After William Scully, owner of the unlicensed pharmaceutical importer Medical Device King/Pharmalogical, was convicted on charges relating to his drug importation business, Bill Swail, host…
During PSM’s 2014 Interchange, Howard Sklamberg, the FDA’s Deputy Commissioner for Global Regulatory Operations and Policy gave an overview of the FDA’s work in protecting the safe drug supply chain and of legislation enacted in 2012 and 2013.
Recently, conversations and debate about drug importation has reached a fever pitch. Partnership For Safe Medicines’ many coalition members want Congress to know about our concerns for the safety of American patients. The secure American drug supply chain protects American consumers from dangerous counterfeit, substandard and unsafe medicines. Any effort designed to bypass FDA controls…
As a coalition whose outreach and operations involve educating health care workers and patients in all fifty states, and whose membership is comprised of more than 60 organizations committed to the safety of prescription drugs and protecting U.S. consumers against counterfeit, substandard or otherwise unsafe medicines, we are deeply concerned that importation proposals will undermine America’s existing and proposed drug safety protocols.
Hear Senior Science Advisor Eric Sampson talk about the CDC Foundation’s plans to study the impact of counterfeit drugs and educate the public health community about the black market.
Update: On December 13, 2017, the U.S. Court of Appeals for the Second Circuit vacated William Scully’s conviction because a lower court “declined to allow Scully to introduce evidence at his 2015 trial showing he sought legal advice about importing drugs with foreign labels from one of his lawyers.” Ultimately Scully pleaded guilty to one charge of…
At the 2014 Interchange, New spoke about the risk that drug diversion poses to patients who may be treated with painkillers that have been contaminated by addicts, and about the scale of drug diversion in hospitals in the United States.
Ever wonder about what’s behind the news for counterfeit drug crime? The Rogues Gallery Comic Book Series tells the real-life stories of fake drug criminals and their cases.
The Partnership for Safe Medicines released the following statement regarding the verdict of William Scully for operating an illegitimate drug supply company called Pharmalogical Inc., which conducts business as Medical Device King. The defendant sold more than $14 million worth of illegal products including misbranded cancer medicine, unapproved birth control devices, and other medical products…
Update: On December 13, 2017, the U.S. Court of Appeals for the Second Circuit vacated William Scully’s conviction because a lower court “declined to allow Scully to introduce evidence at his 2015 trial showing he sought legal advice about importing drugs with foreign labels from one of his lawyers.” Ultimately Scully pleaded guilty to one charge of…
This is a reprint of an FDA Alert. FDA posts press releases and other notices of recalls and market withdrawals from the firms involved as a service to consumers, the media, and other interested parties. FDA does not endorse either the product or the company. For Immediate Release November 9, 2015 Contact Consumers …
“Canadadrugs.com and their co-conspirators are charged with shipping $78 million of counterfeit and unapproved medicines to unsuspecting patients across the United States,” said Thomas Kubic, PSM board member and former FBI Deputy Assistant Director who spent 30 years at our nation’s top law enforcement agency. “Yet to this day and since 2002, Canadadrugs.com has been listed as an “approved” and “safe” online pharmacy by a leading U.S. firm whose business model involves helping Americans unknowingly break the law and put their welfare at risk.
At PSM’s 2014 Interchange, Jim Dahl, a 30 year veteran of Federal law enforcement, spoke about the scale of the counterfeit drug problem in the United States and how much risk Americans should be willing to accept.
Between 2004 and 2009 a number of states experimented with drug importation from countries that supposedly had online pharmacies offering cheaper medications. Illinois was a leader in this area, spending significant state resources marketing and promoting the program while working with Ram Kamath, who later became Director of Pharmacy Policy and International Verifications at PharmacyChecker. The…
Prescription medication can be expensive, but there are safe systems in place to help Americans buy reliable medications at lower prices. Saving at the Doctor’s Office Lower cost medications can start at the doctor’s office. Generic medications are typically just as effective as their namebrand alternatives and they can save patients substantial money. If a…
By February of 2015 the law had been thrown out, but only after the President of the Maine Pharmacy Association experienced first hand what patients can be exposed to when buying drugs from a Canadian online pharmacy. On June 27, 2013, the Maine Legislature passed LD 171, the Act to Facilitate Personal Importation of Prescription…
Update: On December 13, 2017, the U.S. Court of Appeals for the Second Circuit vacated William Scully’s conviction because a lower court “declined to allow Scully to introduce evidence at his 2015 trial showing he sought legal advice about importing drugs with foreign labels from one of his lawyers.” Ultimately Scully pleaded guilty to one charge of…
The Partnership for Safe Medicines released the following statement on the sentencing of Daniel Sanchez for his role in an illegal online counterfeit medicines scheme based in Pittsburgh and Houston: “Drug counterfeiters take advantage of our vulnerable citizens on a daily basis, and have grown globally into a multibillion dollar industry,” said PSM President Marv Shepherd. “This…
The FDA has been prosecuting doctors and unlicensed drug distributors for importing non-FDA approved cancer drugs. Some of those drugs have been counterfeits. Learn more about how to detect counterfeit medication by reading our 8-Step Checklist For Medicine Safety and about recent counterfeit cancer drug prosecutions by reading PSM’s Black Market Cancer Drug Cases, 2007-2013.
Aren’t Canadian medicines ordered online as safe as ours? Well, no. Buying medicines from online pharmacies that claim to be selling Canadian drugs is dangerous.
Ram Kamath has had a single charge of smuggling dropped and is now cooperating with the authorities. Kamath was one of 14 people charged in the case USA vs. CanadaDrugs. Kamath had been accused of participating in the international drug smuggling conspiracy allegedly perpetrated by CanadaDrugs.
Dr. Mohamad Ayman Ghraowi has filed suit against Canada Drugs subsidiaries Montana Healthcare Solutions and Rockeley Ventures, for supplying his practice with counterfeit cancer medication, the Courthouse News Service (CNS) reports.
A change to current laws allows the Food and Drug Administration (FDA) to destroy misbranded medications blocked from entering the United States. Prior to this, the FDA was forced to return such drugs to the shipper.
A Missouri doctor was sentenced for misbranded drugs charges and his Patterson Medical Clinic was sentenced for false statements charges on September 2nd, 2015 related to the purchases of non-FDA approved osteoporosis treatments. Both the doctor and his clinic face 3 years probation.
Medicine approved by the U.S. Food and Drug Administration’s (FDA) comprehensive drug approval process are considered the safest in the world. The U.S.’s closed distribution system keeps medicine counterfeiters and fakers out of pharmacies and away from patients. Medicines in countries without the systemic oversight of an agency like the FDA suffer from pervasive counterfeit…
Michigan pharmacy owner and 18 of his employees face jail time for conspiracies that provided misbranded, returned, and out-of-date pharmaceuticals to nursing homes and adult foster care homes throughout the state.
A Canadian pharmaceutical company and their drop-shipper were fined $45 million, as well as forfeiting $30 million for smuggling Non-FDA approved medications into the U.S. 5 men from Canada and the United States have also pleaded guilty to a multi-year conspiracy to ship misbranded and non-FDA approved pharmaceuticals to U.S. Clinics and Doctors’ Offices.
Securing Industry reports that a new anti-counterfeiting product focused on consumers has been launched to help patients verify the authenticity of their medications. The product, Check My Meds, is a cell phone application that scans two dimensional barcodes on medicine packages to verify their origin in conjunction with new FDA policy. The U.S. Food and…