The Experts Agree: We Need to Protect Our Drug Supply
Pharmacists, patient advocates, regulators, law enforcement and pharmaceutical companies and wholesalers share our concerns about the threat counterfeit drugs pose to American patients.
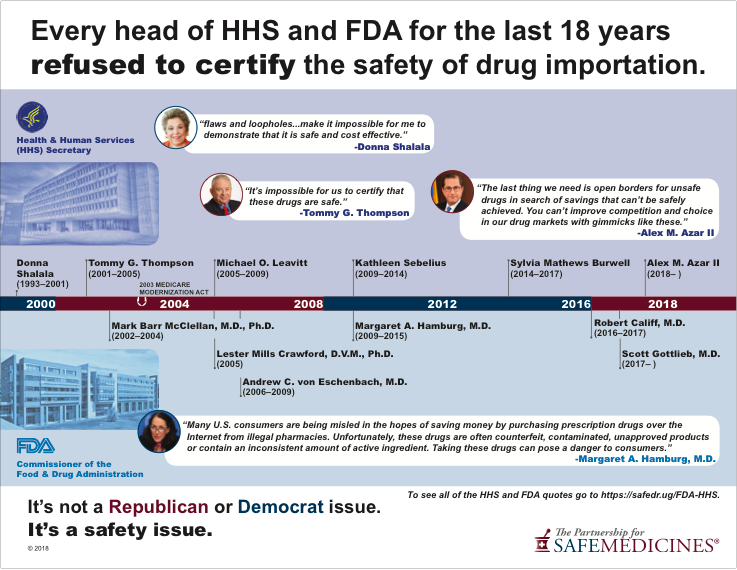
Since 2000, not one head of the U.S. Department of Health and Human Services or one FDA Commissioner—no matter who appointed them—has been willing to certify the importation of drugs from other countries' supplies. As recently as May 2018, Secretary of Health and Human Services Alex Azar II remarked:
...the last four FDA commissioners have said there is no effective way to ensure drugs coming from Canada really are coming from Canada, rather than being routed from, say, a counterfeit factory in China. The United States has the safest regulatory system in the world. The last thing we need is open borders for unsafe drugs in search of savings that cannot be safely achieved.
Read collected editorials from experts addressing the problem of counterfeit medicines.
Read materials from congressional briefings PSM assembles to have experts update Congress about current counterfeit threats.
Watch our video updates and read supporting information about medicine safety issues.
Read the Interchange archives to learn what representatives from government, industry, medicine, science, and patient advocacy have shared about the counterfeit drug trade.