Posts Tagged ‘importation’
Canada doesn’t have track and trace
Canada doesn’t have track and trace January 2023 Download our factsheet about how Canadian importation breaks the U.S. track and trace system.
[...]“Reducing costs requires more than all of this non–value-added activity,” Expert Says
In this October 23, 2020 editorial, which was published in the The American Journal of Managed Care, Michael Abrams explains why drug importation will not save patients money. Abrams is the co-founder and managing partner of Numerof & Associates.
[...]July 29, 2020 video: Importation Executive Order Explainer
On July 24, 2020, the White House issued an executive order to implement three approaches to foreign drug importation, all of which involve dipping into other countries’ drug supplies and putting them in U.S. medicine cabinets. Watch this week’s video to learn more.
[...]July 15, 2020 video: The Slow Boat to Justice–Prosecuting Foreign Drug Counterfeiters
Importing medicine from Canada is more dangerous than it looks because it is so difficult to prosecute fake or substandard drug sellers. Legislators and their consultants make it sound as if holding a foreign vendor accountable will be easy, but history has shown that the U.S. federal government, with all of its agreements, power, and resources, still struggles to bring criminals to justice.
[...]HHS comments come in overwhelmingly against Canadian drug importation proposal
Yesterday ended a 78-day comment period for the White House’s proposal to import medicine from Canada. In all, over 1,000 comments were filed. Overwhelmingly, these comments opposed the proposed rule or expressed skepticism that the rule could meet the two requirements listed in the Medicare Modernization Act of 2003: be safe and save consumers money. In fact, when you read the comments, it is clear that this policy is overwhelming opposed by experts on the issues of economics and medicine safety.
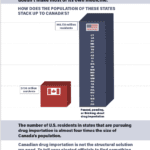
[...]Canadian Importation Does Not Work
The number of U.S. residents in states that are considering importing drugs from Canada is almost four times Canada’s own population. Canadian drug importation is not the structural solution we need.
[...]Myth: We are getting the same drugs Canadians take
In 2014, during a two-year period when Maine was experimenting with drug importation, the president of Maine’s Pharmacy Association purchased medications from an online pharmacy for testing. The drugs he received were not approved for the Canadian or U.S. markets. Worse, they were poor quality: two of them were the wrong strength, and the other was contaminated.
[...]Who Opposes Drug Importation? Every Head of the FDA and HHS Since 2000
Ordering prescription drugs from non-FDA approved foreign sources is a dangerous pat, opposed by all of the previous heads of the United States Food and Drug Administration (FDA) and United States Department of Health and Human Services (HHS) since 2000.
[...]President of the International AntiCounterfeiting Coalition Warns That Importation Will Open the Door to Drug Counterfeiters
In the midst of a nationwide epidemic of opioid addiction fueled by illicit smuggling of drugs from overseas, and coming on the heels of a year in which U.S. Customs and Border Protection seized over $73 million worth of counterfeit medicines at our nation’s ports, some members of Congress have suggested a novel approach to these growing threats: “opening the floodgates.”
[...]Op-Ed: Why Drug Importation Is Flawed Policy
Mike Leavitt | March 20, 2017 With the issue of prescription drug importation being debated on Capitol Hill again, mark me in the skeptical camp. As a matter of safety and practical policymaking, drug importation simply doesn’t work. It is not by happenstance that our country has the world’s safest drug supply. Counterfeit medicines are proliferating…
[...]